Abstract
Background
We explored the association of 29 previously reported neonatal, perinatal, and prenatal conditions, and exposures with later diagnosis of autism spectrum disorder (ASD) in a large sample of children followed over multiple years.
Methods
A retrospective case–cohort study was formed using the Military Health System database. Cases were identified by International Classification of Diseases, Ninth Revision codes for ASD between 2000 and 2013, and were matched 3:1 with controls on sex, date of birth, and enrollment time frame. Exposures included 29 conditions previously associated with ASD; 17 prenatal conditions and their pharmaceutical treatment, 5 perinatal conditions, and 6 neonatal conditions.
Results
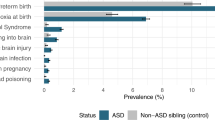
A total of 8,760 children diagnosed with ASD between the ages of 2 and 18 years were matched with 26,280 controls. ASD is associated with maternal mental illness, epilepsy, obesity, hypertension, diabetes, polycystic ovary syndrome, infection, asthma, assisted fertility, hyperemesis, younger maternal age, labor complications, low birth weight, infant infection, epilepsy, birth asphyxia, and newborn complications. The greatest increased risk was associated with infant epilepsy (odds ratio (OR) 7.57 (5.68–10.07)), maternal mental health (OR 1.80 (1.65–1.96)), and epilepsy (OR 1.60 (1.02–2.50)) medications.
Conclusion
ASD is associated with a range of prenatal, perinatal, and neonatal factors, with the highest magnitude associations with maternal medication use and neonatal seizure.
Similar content being viewed by others
Autism spectrum disorders (ASDs) are a group of neurodevelopmental disorders defined by communication and social difficulties, and repetitive behaviors. ASD prevalence has been increasing and is currently estimated at 1 in 68 in the United States (1). Although autism’s etiology has remained elusive, studies documenting a higher concordance in monozygotic vs. dizygotic twins, and increased concordance in siblings suggest a genetic component (1). The imperfect concordance of ASD in identical twins suggests an early-life environmental component to ASD. Numerous prenatal, perinatal, and neonatal factors proposed as drivers of increased risk have been examined in isolation which impacts their validity.
Research into prenatal factors has focused on broad areas of psychiatric and neurological conditions, overweight status and inflammation, autoimmune reaction, and pregnancy-specific conditions. Studies associating maternal epilepsy (2), substance use/abuse (3), mental health conditions (4,5,6,7,8), and treatment with sodium valproate (9), neuroleptics (10), and selective serotonin reuptake inhibitors (11) have endorsed the notion that ASD is related to a genetic maternal brain dysfunction, or that medication capable of impacting the maternal brain would adversely impact the developing brain and increase ASD risk in the offspring. Maternal obesity and inflammation, including overweight/obesity (6,12), diabetes (13,14,15), hypertension (6,14,16,17), polycystic ovary syndrome (PCOS) (18), and infections (4,8,19) have been associated with ASD. Researchers hypothesize that adiposity-induced inflammation and infection-associated inflammation increases the risk (20). Autoimmune theories have postulated that the autoimmune reaction adversely impacted the developing fetus. Research has focused on linkages between ASD and hypothyroidism (21), asthma (22) and its treatment (23), celiac, and autoimmune disease (15). Research into pregnancy factors, including fertility treatment (24), hyperemesis (4,14), anemia (2), medication use in pregnancy (13,14,25), and maternal age (4,6,7,8,13,14,16,17,26,27) have also found significant associations, suggesting that something goes wrong during the pregnancy driving ASD risk. However, links between these risk factors and ASD are inconsistent across studies (19), covariates vary widely, studies rarely examine the impact of medications to treat the studied conditions, and when medications are included, their relationship to diagnose conditions is not examined. In addition, researched conditions within the same area are often connected and overlap. Examination of one or another condition without accounting for others makes it especially difficult to tease out the effects of related but separate conditions.
Perinatal research has focused on possible trauma during the birth process, with studies linking ASD with multiple gestation (28), pregnancy complications (4,8,13,17,26,29), preterm birth (4,7,8,14,27), post-term birth (30), and labor complications, including induced labor (29,31), cesarean section (29,31), breech presentation (14,26,28), fetal distress (28,29), postpartum hemorrhage (29), and prolonged labor (8,28). Similarly, newborn research has concentrated on neonatal distress, including low birth weight (LBW) (4,7,27,28,32), birth asphyxia (8,16,25,30,33), infections (33), epilepsy (33), neonatal complications (4,25,27,31,33), and jaundice (8,14,28,30,34). Similar to studies of prenatal risk, results from studies of perinatal and neonatal associations are inconsistent, and covariates vary widely.
Inconsistent results likely relate to smaller sample sizes (8,16,19,22,26) and narrow evaluation of other identified factors (5,11,23). Although numerous ASD associations have been identified, their examination in isolation increases the likelihood that studies miss unknown confounders. We theorize that ASD risk factors have been overidentified as many studies may be detecting associations with hypothesized ASD without adequately addressing confounding of other known or suspected risk factors. We hypothesize that simultaneous examination of 29 previously reported prenatal, perinatal, and neonatal risk factors for ASD will identify a more discrete set of associated factors.
Methods
A retrospective case cohort was formed using the Military Health System database. This database includes records of care provided to uniformed services’ members and their family members at military and civilian healthcare facilities domestically and abroad. Cases of ASD were defined utilizing a previously validated methods that has been widely used. Cases included children diagnosed with ASD between the age of 2 and 18 years, with an International Classification of Diseases, Ninth Revision (ICD-9) diagnostic code for ASD at two separate encounters between 1 October 2000 and 30 September 2013. The exclusion criteria for both cases and controls included diagnosed childhood disintegrative disorder, and diagnosis with ICD-9 code 330.8 (childhood disintegrative disorder), which includes Rett syndrome. Controls with a single diagnosis of ASD were also excluded (35). All cases and controls were born in the Military Health System and their mothers were followed for at least 1 year before the child’s birth. Three controls were matched to each case by age, sex, and the child’s enrollment time frame.
For cases and controls, ICD-9 codes identified prenatal diagnoses in mothers’ in-patient and outpatient record; identification time period differed by diagnosis (Supplementary Table 1). Mothers’ ambulatory pharmaceutical records identified medications prescribed during the 3 months preceding pregnancy and the pregnancy period (Supplementary Table 1). Pregnancy length was calculated using birth record gestational age. In-patient prescription data were unavailable. Medications were classified as corresponding with the given medical conditions (e.g., antibiotic use with infection; insulin with diabetes). Infection, hypertension, diabetes, asthma, mental illness, epilepsy, PCOS, hyperemesis, hypothyroidism, and infertility are commonly treated pharmaceutically, yet mothers may curtail medication use during pregnancy. To account for off-label prescriptions, and explore the differences in ASD association related to diagnosis of conditions as opposed to their pharmaceutical treatment, variables were coded with four levels: (1) no disease/no ICD-9 diagnosis; (2) disease diagnosis alone; (3) diagnosis with associated prescription; and (4) prescription medication only. Obesity, anemia, autoimmune, celiac, and substance-abuse diagnoses are not commonly treated pharmaceutically and were dichotomized, as was muscle relaxant use, which does not have a corresponding diagnosis. Mother’s active-duty status was recorded, and maternal age was categorized as <25, 25–35, and >35 years.
Perinatal conditions were identified by ICD-9 codes in maternal and infant in-patient and outpatient medical records. Neonatal conditions were identified by ICD-9 codes in infants’ in-patient and outpatient medical records for the first 90 days of life to account for infants with longer birth hospitalizations and with diagnoses recorded at the time of discharge instead of the time of recognition (Supplementary Table 1). Neither perinatal nor neonatal conditions were linked to medication data. The Healthcare Cost and Utilization Project Clinical Classification Software was used to categorize ICD-9 codes; codes for developmental delays and substance-abuse disorders were removed from Clinical Classification category -5, the mental health category. Neonatal jaundice was identified by ICD-9 codes and phototherapy procedure codes.
Additional covariates included the mother’s active-duty status since service requirements may make active-duty women healthier than mothers who are married to active-duty members. A variable was added to the model with a count of total maternal health-care visits in the year before the child’s birth. This count variable was added to adjust for potential confounding from health-care-seeking behavior as frequent contact with medical providers may increase the identification and diagnosis of medical conditions such as ASD.
Variable selection using the shrinkage method was performed using Lasso conditional logistic regression. Lasso was performed using 10-fold cross-validation to select a shrinkage parameter that would optimize outcome prediction and minimize cross-validation deviance. Following the exclusion of variables with Lasso, conditional logistic regression analysis calculated unadjusted and adjusted odds ratio (OR) with 95% confidence intervals (CIs) of ASD. To ensure stringent selection of variables, a P value adjustment method was applied to control the false discovery rate. We considered false discovery rate <0.05 to be significant. Tetrachoric correlation was measured between birth asphyxia and neonatal seizure to confirm that neonatal seizure was linked with birth asphyxia. For univariate analysis, means and standard deviations were used for normally distributed data, and medians and interquartile ranges for data that were not normally distributed. Analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC), and R 3.4 using the clogitL1 package (R Foundation for Statistical Computing, Vienna, Austria). The study was reviewed and approved by the responsible institutional review board.
Results
A total of 8,760 infants born in the Military Health System with diagnosed ASD were matched with 26,280 controls by sex (79.9% male), and date of birth (99.9%). When not matched on the exact date of birth, the mean difference was 9.6 days. Mothers of cases and controls differed on age and active-duty status (Table 1).
Maternal Associations
In unadjusted analysis, odds of ASD were increased with maternal hypertension, asthma, mental illness, PCOS, hyperemesis, and assisted fertility as identified by diagnosis alone, diagnosis with pharmaceutical treatment, and pharmaceutical treatment alone. ASD was associated with maternal pharmaceutical treatment for infection, diabetes, and epilepsy, but not diagnosis for these conditions without prescription medications. Diagnosed hypothyroidism with pharmaceutical treatment, autoimmune disease, maternal age of <25 years, obesity, diagnosed substance abuse, and muscle relaxant use was associated with children’s increased ASD risk. Hypothyroidism without pharmaceutical treatment, and maternal age of 35+ years were associated with decreased ASD risk. Anemia, celiac disease, pharmaceutical treatment for hypothyroidism without diagnosis, and diagnosed epilepsy, diabetes, and infection without pharmaceutical treatment were not associated with ASD (Table 2). Maternal active-duty status was associated with decreased ASD risk, and increased maternal health-care contacts were associated with increased risk.
The cross-validated Lasso conditional logistic regression resulted in the selection of 21 variables in the model. After false discovery rate adjustment, 19 variables remained in the model, with some levels of multilevel variables proving to be nonsignificant. In this adjusted analysis, odds of ASD increased with asthma and hyperemesis as identified by diagnosis, diagnosis with pharmaceutical treatment, and pharmaceutical treatment alone. ASD was increased with maternal pharmaceutical treatment (with or without diagnosis) for infection, diabetes, mental illness, and epilepsy, but not diagnosis of these conditions without pharmaceutical treatment. ASD was increased with hypertension diagnosis and diagnosis with treatment, but not treatment alone. Medications associated with treatment of PCOS (primarily norethindrone birth control; Table 3) were associated with ASD, but only in the absence of a PCOS diagnosis. Infertility was associated with ASD only when both diagnosis and pharmaceutical infertility treatments were present. Maternal age of <25 years and obesity were associated with ASD. Maternal active-duty status, hypothyroidism diagnosis without pharmaceutical treatment, and maternal age >35 years were associated with decreased odds of ASD (Table 2).
Perinatal Associations
In unadjusted analysis, multiple gestation, pregnancy complications, labor complications, preterm birth, and LBW were associated with ASD. Post-term birth and ASD were not associated. In adjusted analysis, only labor complications and LBW were associated with ASD (Table 2).
Neonatal Associations
Neonatal factors associated with ASD in unadjusted analysis included jaundice, infection, epilepsy, birth asphyxia, and newborn complications (Table 2). These associations remained after adjustment with the exception of jaundice, which became nonsignificant. Neonatal seizure conferred the greatest increased odds of ASD (Table 2) and was correlated with birth asphyxia (P=0.027).
Discussion
In an analysis of 29 previously identified risk factors for ASD in a large pediatric population using techniques to minimize false discovery rates, only 19 of those risk factors had associations with a diagnosis of ASD. Of 10 maternal prenatal conditions commonly treated pharmaceutically, we found diagnosis alone not to be associated with ASD for six of the identified conditions (infections, diabetes, mental illness, PCOS, assisted fertility, and epilepsy), but treatment for those conditions was associated. Pharmaceutical treatment may be an indicator of disease severity or may suggest that the medication instead of the underlying disease is associated with increased risk of ASD in the offspring. Increased risk with medication use (regardless of whether a diagnosis was made) and no association with diagnosis alone may point to medication being an important factor.
Prenatal Factors
Maternal mental health conditions, epilepsy, and substance abuse have been linked with children’s ASD (2,3,4,5,6,7,8), as have smaller studies examining pharmaceutical treatment of these conditions (9,10,11). In our population, ASD was not associated with maternal mental health or epilepsy diagnoses without medication use. However, pharmaceutical treatment with and without a corresponding diagnosis was associated with ASD. Small studies have previously linked ASD with selective serotonin reuptake inhibitors (11); however, other studies have found no link between maternal psychotropic medications and ASD (5). Valproate, which accounted for 18% of antiepileptics prescribed for mothers with epilepsy and 6% of antiepileptics for mothers without diagnosed epilepsy, has been linked with ASD (9). Diagnosed substance abuse was associated with ASD in unadjusted, but not adjusted analysis. Cyclobenzaprine, which accounted for 79% of muscle relaxant prescriptions, was introduced as an antidepressant and blocked serotonin transporters; however, in adjusted analysis, muscle relaxant use was not associated with ASD.
The lack of association of these diagnoses and one medication in this large case–control cohort study is likely due to the impact of other factors studied. Findings of increased risk associated with mental health and epilepsy medications may relate to medication use acting as an indicator of disease severity, but may also suggest that medications for these conditions have an impact on the developing fetus. It is biologically plausible that medications that target the adult brain can have an impact on the developing brain. The targeted study of specific medications, dosage, trimester of exposure, and the relationship with diagnoses is needed to better understand this association.
Research has associated obesity and its related conditions with ASD (6,12) with the hypothesis that adiposity-induced inflammation increases the risk (20). Our results corroborate the connection, yet methods preclude the elucidation of the mechanisms of risk related to weight gain, preexisting obesity, or adiposity and inflammation (12). Prenatal diabetes and ASD have been linked (13,14,15); theorized mechanisms of action include hypoglycemia-induced fetal hypoxia, oxidative stress, and autoimmunity related to type I diabetes (36). We found no association between ASD and diagnosed diabetes that was not pharmaceutically treated, but medication use (with and without diagnosis) was associated with ASD. Pharmaceutical treatment without diagnosis was predominately associated with type II diabetes medications; increases may indicate disease severity, or noncompliance with diet and exercise recommendations. Pharmaceutical treatments associated with a diabetes diagnosis were predominately insulin prescriptions (Table 3). Again, this pattern suggests severe disease requiring prescription medication that increases ASD risk, as pharmaceutical treatment and disease severity are linked. Our finding linking diagnosed hypertension (with and without pharmaceutical treatment) with ASD in adjusted analysis is consistent with previous research (6,14,16,17). Results indicating no link between hypertension medication use alone and ASD suggest that the hypertensive state and not antihypertensive treatment is associated with ASD. Findings that PCOS diagnosis and diagnosis with treatment were not associated with ASD contradict previous research (18), and may indicate that previous research did not account for confounders. Hormone treatment without diagnosed PCOS is consistent with progestin use to increase early-pregnancy viability and aid the preterm fetus in later pregnancy, supporting a possible explanation for the reported association between pregnancy complications and ASD. Maternal prenatal infection has been linked with ASD (37), with inflammation as a hypothesized mechanism of action. In our study, 58% of included mothers had infection or antibiotic medication use during pregnancy. Diagnosed infection was not associated with ASD, but pharmaceutical treatment (largely with antibiotics; Table 3) was associated with ASD. Findings may indicate severe infection, requiring medication increases the risk, or that pharmaceutical treatments are the more salient ASD risk factor. It is possible that the immune activation increases risk, or that antibiotic treatment negatively impacts the microbiome, which has also been linked with autism. Further research is needed to clarify the complex relationship between infection, treatment, and ASD in the offspring.
Research linking asthma, hypothyroidism, and celiac disease with ASD suggests that inflammation and immune dysregulation may increase ASD risk (15,21,22). ASD has been linked with maternal asthma (22) and β-2 adrenergic agonist use (23). We found that asthma diagnosis and diagnosis with pharmaceutical treatment are associated with ASD (Table 3), possibly suggesting that atopy and ASD genes are related. Terbutaline prescriptions in the pharmaceutical treatment group (Table 3) are likely related to preterm labor and not asthma. Maternal hypothyroidism alone was associated with decreased ASD risk in our study, whereas treatment (with and without diagnosis) was not associated with ASD in adjusted analysis. Previous research linked ASD in children with maternal thyroid peroxidase antibody (23), low free thyroxine during pregnancy (38), and hyper- and hypothyroidism following pregnancy (34), but not with thyroid hormone levels (21), or treated hyper- or hypothyroidism during pregnancy (34). Our finding is consistent with this previous research. It is unclear why diagnosed hypothyroidism without medication was protective; perhaps, these cases are borderline and trigger increased pregnancy monitoring, which proves to be protective through early identification of other risk factors. Autoimmune and celiac disease were not significantly associated with ASD in adjusted or unadjusted analyses, providing evidence against previous reports of associations.
In unadjusted analysis, all indicators of infertility were associated with ASD; in adjusted analysis, only fertility diagnosis with medication was significantly associated. Previous research on infertility and ASD is similarly inconsistent (24,39). These findings suggest that the specific underlying causes of infertility or severe infertility requiring medication may increase ASD risk (Table 2). Common comorbidities of pregnancy, including hyperemesis and anemia have been inconclusively linked with ASD (2,4,14). In our unadjusted and adjusted models, maternal hyperemesis identified by diagnosis, diagnosis with treatment, and pharmaceutical treatment alone was associated with ASD. Pharmaceutical treatment of hyperemesis may contribute to ASD risk, as may hyperemesis itself, via early-pregnancy micronutrient malnutrition which may have an impact on fetal brain development. ASD was not associated with anemia in adjusted or unadjusted models.
Contrary to considerable previous research (8,13,14,16,17,26,27), we found that older maternal age was associated with decreased odds of ASD, and younger maternal age was associated with increased odds of ASD in adjusted and unadjusted analyses. Results may relate to the relatively homogeneous age of included mothers, Military Health System access to care specifics, superior health of active-duty women, age grouping parameters, or associations between age and income, as military rank and income generally increase with age (Table 2). We found that maternal active-duty military service was associated with decreased odds of ASD. The results might relate to better health in active duty vs. civilian spouses or income.
Perinatal and Neonatal Factors
Using a larger sample than previous studies, and after accounting for multiple other risk factors, this study corroborates previous research linking labor complications (8,14,26,28,29,31) and LBW (4,7,27,28,32) with ASD. The findings support the concept that increasing ASD prevalence may partially relate to improved survival of infants born with LBW and with complications. Multiple gestation, pregnancy complications, and preterm birth were not significantly associated with ASD in adjusted models, likely related to their close correlations with the significant outcomes of LBW and labor complications.
Neonatal jaundice and ASD were not associated in our adjusted model, despite research supporting the connection (8,14,28,30,34). The results mirror recent research, finding no association between ASD and hyperbilirubinemia identified by bilirubin laboratory values (40), and suggest that confounders have an impact on previous studies linking jaundice and ASD.
Although neonatal seizure had an impact on few children, it was associated with over seven times the odds of ASD consistent with smaller previous studies (33). The results were likely related to difficulties with delivery and resultant brain injury; over 50% of infants with neonatal seizure had birth asphyxia, suggesting the need for close monitoring of infants at risk. Consistent with previous research, ASD was significantly associated with neonatal infection (33), birth asphyxia (8,16,25,30,33), and newborn complications (4,25,27,31,33) in adjusted analysis. The findings indicate the importance of neonatal health in ASD prevention, and support the theory that increasing ASD prevalence may be related to survival of higher risk infants.
Limitations
The limitations of our study include reliance on ICD-9 diagnosis codes. Our data also use medication prescriptions and cannot document medicine use or duration. With large data sets and analyses examining multiple potential associations, the risk of type 1 error, or false positives, exists. We attempted to address this risk by using both the Lasso method and strategies to reduce the false-discovery rate. Although some of our results contradict the previous findings, most of them reported here are consistent with previous literature. Findings at odds with previous reports are largely nonsignificant findings in our study that can be explained by our large inclusive model, including the previously unmeasured confounders. The inclusion of many variables also prevented in-depth examination of the effects of specific medications, or the impact of medication use by trimester. The design, however, did provide an inclusive picture of the interplay between diagnoses and medication. Finally, we did not have access to demographic data on mothers other than age, making us unable to control for maternal education, race, and marital status.
The strengths of our study include the use of multiple prenatal, perinatal, and neonatal risk factors, and inclusion of medication use before and during pregnancy. We were able to follow mothers for a year before children’s birth, use confirmed diagnoses that reduced recall bias, and follow children for long periods to correctly classify children diagnosed at older ages. Finally, our large study population exceeds the size of populations of other ASD risk studies, including the combined populations of many meta-analyses.
Conclusion
Within a large representative sample, we confirmed the associations between ASD and multiple prenatal, perinatal, and neonatal factors. Associations between ASD and neonatal brain injury, maternal medication use, and maternal age warrant further exploration. Our results support the theory that the genesis of ASD is multifactorial; while a single factor may increase the risk, it is unlikely that a single unifying exposure will explain the risk across the spectrum.
References
Prevention, C.f.D.C.aAutism Spectrum Disorder. Data & Statistics 2016 March 31, 2016 [cited 2016 May 19, 2016]; Available at http://www.cdc.gov/ncbddd/autism/data.html.
Leonard H, de Klerk N, Bourke J, Bower C. Maternal health in pregnancy and intellectual disability in the offspring: a population-based study. Ann Epidemiol 2006;16:448–454.
Davis E, Fennoy I, Laraque D, Kanem N, Brown G, Mitchell J. Autism and developmental abnormalities in children with perinatal cocaine exposure. J Natl Med Assoc 1992;84:315–319.
Zhang X, Lv CC, Tian J et al. Prenatal and perinatal risk factors for autism in China. J Autism Dev Disord 2010;40:1311–1321.
Sorensen MJ, Grønborg TK, Christensen J et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol 2013;5:449–459.
Dodds L, Fell DB, Shea S, Armson BA, Allen AC, Bryson S. The role of prenatal, obstetric and neonatal factors in the development of autism. J Autism Dev Disord 2011;41:891–902.
Larsson HJ, Eaton WW, Madsen KM et al. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol 2005;161:916–925 discussion 926–928.
Duan G, Yao M, Ma Y, Zhang W. Perinatal and background risk factors for childhood autism in central China. Psychiatry Res 2014;220:410–417.
Wood AG, Nadebaum C, Anderson V et al. Prospective assessment of autism traits in children exposed to antiepileptic drugs during pregnancy. Epilepsia 2015;56:1047–1055.
Eriksson MA, Westerlund J, Anderlid BM, Gillberg C, Fernell E. First-degree relatives of young children with autism spectrum disorders: some gender aspects. Res Dev Disabil 2012;33:1642–1648.
Gidaya NB, Lee BK, Burstyn I, Yudell M, Mortensen EL, Newschaffer CJ. In utero exposure to selective serotonin reuptake inhibitors and risk for autism spectrum disorder. J Autism Dev Disord 2014;44:2558–2567.
Gardner RM, Lee BK, Magnusson C et al. Maternal body mass index during early pregnancy, gestational weight gain, and risk of autism spectrum disorders: results from a Swedish total population and discordant sibling study. Int J Epidemiol 2015;44:870–883.
Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry 2009;195:7–14.
Guinchat V, Thorsen P, Laurent C, Cans C, Bodeau N, Cohen D. Pre-, peri- and neonatal risk factors for autism. Acta Obstet Gynecol Scand 2012;91:287–300.
Atladottir HO, Pedersen MG, Thorsen P et al. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics 2009;124:687–694.
Mamidala MP, Polinedi A, PK PTV et al. Prenatal, perinatal and neonatal risk factors of Autism Spectrum Disorder: a comprehensive epidemiological assessment from India. Res Dev Disabil 2013;34:3004–3013.
Burstyn I, Sithole F, Zwaigenbaum L. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Dis Can 2010;30:125–134.
Kosidou K, Dalman C, Widman L et al. Maternal polycystic ovary syndrome and the risk of autism spectrum disorders in the offspring: a population-based nationwide study in Sweden. Mol Psychiatry 2015;21:1441–1448.
Atladottir HO, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics 2012;130:e1447–e1454.
Li M, Fallin MD, Riley A et al. The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics 2016;137:e20152206.
Brown AS, Surcel HM, Hinkka-Yli-Salomäki S, Cheslack-Postava K, Bao Y, Sourander A. Maternal thyroid autoantibody and elevated risk of autism in a national birth cohort. Prog Neuropsychopharmacol Biol Psychiatry 2015;57:86–92.
Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case–control study. Arch Pediatr Adolesc Med 2005;159:151–157.
Gidaya NB, Lee BK, Burstyn I, Michael Y, Newschaffer CJ, Mortensen EL. In utero exposure to beta-2-adrenergic receptor agonist drugs and risk for autism spectrum disorders. Pediatrics 2016;137:e20151316.
Mamidala MP, Polinedi A, Kumar PT et al. Maternal hormonal interventions as a risk factor for Autism Spectrum Disorder: an epidemiological assessment from India. J Biosci 2013;38:887–892.
Froehlich-Santino W, Londono Tobon A, Cleveland S et al. Prenatal and perinatal risk factors in a twin study of autism spectrm disorders. J Psychiatric Res 2014;54:100–108.
Bilder D, Pinborough-Zimmerman J, Miller J, McMahon W. Prenatal, perinatal, and neonatal factors associated with autism spectrum disorders. Pediatrics 2009;123:1293–1300.
Maimburg RD, Vaeth M. Perinatal risk factors and infantile autism. Acta Psychiatr Scand 2006;114:257–264.
Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics 2011;128:344–355.
Glasson EJ, Bower C, Petterson B, de Klerk N, Chaney G, Hallmayer JF. Perinatal factors and the development of autism: a population study. Arch Gen Psychiatry 2004;61:618–627.
Sugie Y, Sugie H, Fukuda T, Ito M. Neonatal factors in infants with Autistic Disorder and typically developing infants. Autism 2005;9:487–494.
Polo-Kantola P, Lampi KM, Hinkka-Yli-Salomäki S, Gissler M, Brown AS, Sourander A. Obstetric risk factors and autism spectrum disorders in Finland. J Pediatr 2014;164:358–365.
International, I.2014 Demographics Profile of the Military Community, O.o.t.D.A.S.o. Defense, Editor. 2014, Department of Defense. p 200.
Atladottir HO, Schendel DE, Parner ET, Henriksen TB. A descriptive study on the neonatal morbidity profile of autism spectrum disorders, including a comparison with other neurodevelopmental disorders. J Autism Dev Disord 2015;45:2429–2442.
Andersen SL, Laurberg P, Wu CS, Olsen J. Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG 2014;121:1365–1374.
Coleman KJ, Lutsky MA, Yau V et al. Validation of autism spectrum disorder diagnoses in large healthcare systems with electronic medical records. J Autism Dev Disord 2015;45:1989–1996.
Zu G, Jing J, Bowers K, Liu B, Bao W. Maternal diabetes and the risk of autism spectrum disorders in the offspring: a systematic review and meta-analysis. J Autism Dev Disord 2014;44:766–775.
Meldrum SJ, Strunk T, Currie A, Prescott SL, Simmer K, Whitehouse AJ. Autism spectrum disorder in children born preterm-role of exposure to perinatal inflammation. Front Neurosci 2013;7:123.
Roman GC, Ghassabian A, Bongers-Schokking JJ et al. Association of gestational maternal hypothyroxinemia and increased autism risk. Ann Neurol 2013;74:733–742.
Maimburg RD, Vaeth M. Do children born after assisted conception have less risk of developing infantile autism? Hum Reprod 2007;22:1841–1843.
Wu YW, Kuzniewicz MW, Croen L, Walsh EM, McCulloch CE, Newman TB. Risk of autism associated with hyperbilirubinemia and phototherapy. Pediatrics 2016;138:e20161813.
Acknowledgments
This study was conducted with support from the Congressional Directed Medical Research Program, Autism Research Award: W81XWH-12-2-0066 to C.M.N..
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the United States Departments of the Navy, Air Force, Defense, or the US Government. Some authors are a military service member or a US Government employee. This work was prepared as part of their official duties. Title 17 USC 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 USC 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person’s official duties.
Additional information
Fort Belvoir Community Hospital, Fort Belvoir, Virginia, USA
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Hisle-Gorman, E., Susi, A., Stokes, T. et al. Prenatal, perinatal, and neonatal risk factors of autism spectrum disorder. Pediatr Res 84, 190–198 (2018). https://doi.org/10.1038/pr.2018.23
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2018.23
This article is cited by
-
Additive interaction between birth asphyxia and febrile seizures on autism spectrum disorder: a population-based study
Molecular Autism (2024)
-
Role of AI/ML in the Study of Autism Spectrum Disorders: A Bibliometric Analysis
Journal of Technology in Behavioral Science (2024)
-
Mind the NIH-Funding Gap: Structural Discrimination in Physical Health–Related Research for Cognitively Able Autistic Adults
Journal of Autism and Developmental Disorders (2024)
-
Sex-specific and sex-independent steroid-related biomarkers in early second trimester maternal serum associated with autism
Molecular Autism (2023)
-
The association between post-term births and autism spectrum disorders: an updated systematic review and meta-analysis
European Journal of Medical Research (2023)