Abstract
Background:
A vitamin B1-deficient soy-based infant formula was marketed in Israel in 2003, exposing infants to clinical or subclinical B1 deficiency. We investigated whether subclinical B1 deficiency in early infancy had medical, neurodevelopmental, or cognitive effects at 3–5 y of age.
Methods:
A historical prospective cohort study was conducted consisting of four groups: “exposed,” consuming a B1-deficient soy-based formula exclusively for four consecutive weeks or longer; “control,” consuming no soy-based formula; “mixed,” consuming the formula nonexclusively or exclusively for less than four consecutive weeks; and “other,” consuming soy-based formulas other than Remedia. Participants were evaluated by medical examination, Stanford–Binet (SB) intelligence test, sensory profile evaluation, and Conners scales (attention deficit disorder/attention deficit and hyperactivity disorder (ADD/ADHD)).
Results:
Following adjustment for gender, age, and maternal education, there were no significant differences among the four groups on the mean SB scores, on the verbal and nonverbal scores, or in the proportion of children in each group with scores <90. A significantly higher proportion of exposed children as compared with control children had an impaired sensory profile and scores on the Conners scales (ADD/ADHD), but these proportions were also high in the “other” and “mixed” groups.
Conclusion:
The results do not support an association between subclinical B1 deficiency in infancy and long-term development.
Similar content being viewed by others
Main
Vitamin B1 (thiamine) is a water-soluble vitamin. Thiamine occurs in the human body as free thiamine and its phosphorylated forms: thiamine monophosphate, thiamine triphosphate, and thiamine pyrophosphate. In carbohydrate metabolism, thiamine pyrophosphate helps to convert pyruvate to acetyl CoA, which enters the Krebs cycle and is needed for the subsequent steps to generate adenosine triphosphate. Thiamine plays a key role in maintaining memory, the health of the nervous system, and heart muscle and is important for growth, mental development, and learning skills in children (1,2,3). Isolated and characterized in the 1930s, thiamine was the first organic compound to be recognized as a vitamin (1,4,5).
Beriberi, or thiamine deficiency, described in Chinese literature as early as 2600 BC, may result from inadequate thiamine intake, increased requirement for thiamine, excessive loss of thiamine, consumption of antithiamine factors in food, or a combination of these factors. Inadequate consumption is the main cause of thiamine deficiency in underdeveloped countries (1,6,7,8). Breast-fed infants whose mothers are thiamine deficient may develop infantile beriberi. In industrialized countries, the main cause of deficiency is alcoholism, which is associated with low intake of nutrients in general, and thiamine in particular (6,9,10).
Severe vitamin B1 deficiency in infants is almost invariably an acute disease. Onset of symptoms is often rapid and the fatality rate is very high (3). The acute disease is characterized by peripheral neuropathy and muscle weakness, also called “dry” beriberi to differentiate it from “wet” beriberi, which has essentially cardiovascular manifestations (4,5,11). In contrast, the symptoms of subclinical (mild or partial) thiamine deficiency are vague and nonspecific, making it difficult to diagnose. Thiamine deficiency has been associated with sudden infant death syndrome, reduced growth in the young, chronic ill health in young and middle-aged adults, falls and fractures in old age, and impaired reaction to stress in adults (5).
Little is known about the possible long-term neurobehavioral effects of severe, moderate, and mild thiamine deficiency in infancy. The long-term neurobehavioral development of young children is largely dependent on the environment they are exposed to (12,13,14). Intrauterine exposure of the fetus to neuroteratogenic agents such as heroin, cocaine, and alcohol may have deleterious effects on long-term postnatal development (15,16). Postnatal exposure of normal infants to a damaging environment, including nutritional deficiencies especially during the first year of life, may have long-lasting effects similar to those observed following intrauterine exposure (12,17,18,19). Indeed, such long-term developmental consequences have been associated with deficiencies in several vitamins in childhood, including vitamin B12 (20). It is not clear whether mild or subclinical B1 deficiency in infancy, which theoretically could cause slight neurological damage, also has long-term neurodevelopmental sequelae such as motor, cognitive, behavioral, or other changes, including inattention, hyperactivity, and/or learning disability.
In November 2003, following a report of unexplained encephalopathy in a cluster of infants in a tertiary medical center in central Israel, the Israeli Ministry of Health initiated an investigation. The cause was found to be a soy-based infant formula (manufactured by Humana, (Herford, Germany) for distribution in Israel as Remedia) lacking vitamin B1 due to a change in May 2003 in the formula composition (21,22). The deficient formula was apparently sold in Israel for about 6 mo, from May 2003 to 7 November 2003, when the diagnosis of beriberi was established. We report here on the developmental outcome at 3–5 y of age of 216 children exposed exclusively to this diet for at least 1 mo and who had no obvious clinical signs of vitamin B1 deficiency during the critical time of exposure.
Results
General
Of the total 430 participants, 241 (56.0%) were males. This gender distribution was similar (P = 0.669) in all study groups.
Table 1 presents the main characteristics of the study groups. The only significant difference was a higher age at the time of the examination in the exposed group as compared with the control group (4.39 and 4.04 y, respectively, P = 0.005).
Stanford–Binet Intelligence Scale, 5th Edition
Full Stanford–Binet (SB) results were available for 422 participants. Mean scores and SDs of the SB scores are presented in Table 2 . The mean scores in the exposed group were generally lower than those of the control group, but these differences did not reach statistical significance. When compared with the soy-based group, mean scores in the exposed group were generally lower (except for the knowledge subtest), and statistically significant for the quantitative reasoning (QR) subtest (a difference of 6.33 points, P = 0.03). Comparisons of the exposed group with the mixed-nutrition group were inconsistent ( Table 2 ).
The independent impact of the exposure to the B1-deficient formula on these differences was assessed by linear regression models, predicting the scores of the main domains of the SB test, full-scale intelligence quotient (FSIQ), verbal intelligence quotient (VIQ), and nonverbal intelligence quotient (NVIQ), and the QR subtest. On the basis of the preliminary univariate analyses (data not shown), these linear regression models were adjusted for gender, age at examination, and maternal education. Gender and maternal education were significant predictors of the FSIQ, VIQ, and NVIQ scores, whereas study group and age at examination did not significantly impact these scores. For the QR subtest, both maternal education and age at the time of the test were significant predictors whereas gender was insignificant. Being in the exposed vs. soy-based study group remained an independent predictor for the QR score following adjustment ( Table 3 ).
The proportions of SB scores <90 in the various groups are presented in Table 4 . As compared with the control group, the proportions in the exposed group were generally higher, except for the fluid reasoning subtest, but statistically insignificant. As compared with the soy-based group, the proportions in the exposed group were higher and the differences were statistically significant for the VIQ score (7.1 vs. 20.3%, respectively, P = 0.04). Comparisons with the mixed-nutrition group were inconsistent.
To assess the independent impact of exposure to the B1-deficient Remedia formula on lower SB scores, we used logistic regression models, in which a score <90 in the FSIQ, VIQ, and NVIQ domains was the dependent variable, adjusting for gender, maternal education, and maternal smoking, based on preliminary univariate analyses (data not shown). Following adjustment, the exposure to the B1-deficient formula did not significantly impact the results ( Table 5 ).
To explore the potential effect of age at exposure and exposure duration on SB scores <90, an additional analysis was carried out in the exposed group only. Logistic regression models were applied with SB subtest scores <90 as the dependent variables and age at the beginning of the exposure (in weeks) and exposure duration (weeks) as the independent variables, adjusted for gender and maternal education ( Table 6 ). Older age at exposure was associated with higher risk for SB scores <90, albeit not statistically significant; longer exposure duration was significantly associated with higher risk for a score <90 on the working memory (WM) and fluid reasoning subtests ( Table 6 ).
We repeated this analysis while stratifying for maternal education (≤12 and >12 y of schooling). In both strata, neither age at exposure nor exposure duration was associated with the outcomes (data not shown).
Motor Questionnaire
As explained earlier, performance of <90% of the items expected for the age of the child was defined as indicating a problem. Only six children from the total cohort were found positive: 3 (1.6%) in the exposed, 2 (2.1%) in the control, none in the soy-based, and 1 (1.6%) in the mixed-nutrition groups (P value = 0.84).
When walking-initiation age was compared between the study groups, no significant differences were noted: 4 (2.7%), 1 (1.4%), 0 (0%), and 3 (6.8%) children in the exposed, control, soy-based, and mixed-nutrition groups, respectively, initiated walking above the age of 18 mo (P = 0.25).
Sensory Profile
Low compliance of parents in completing the sensory profile questionnaires accounted for the total n of 294, i.e., 70% of the study population. The percentage of children with a score indicating a sensory problem was significantly higher (P = 0.02) in the exposed (13.1%) as compared with the control group (2.8%). The percentages in the other groups consuming soy-based formulas were similar to that of the exposed group: 25% (P = 0.09) in the “soy-based” and 15.2% (P = 0.72) in the mixed-nutrition groups.
Univariate regression models in which impaired sensory profile served as the dependent variable identified no significant correlates. A multiple logistic regression model with the study groups as independent variables was constructed, using first the exposed group and then the control group as the reference category. The results confirmed the significant differences between the three soy-based formula groups (exposed, soy-based, and mixed-nutrition) and the control group, and the statistically insignificant differences between the exposed and the other soy-based groups ( Table 7 ).
The Conners Rating Scales
Not all parents filled out the forms, resulting in lower than expected numbers for each group. As compared with the control group, the proportion of children with a Conners score suggestive of a problem was higher in all three groups consuming soy-based formula: exposed, soy-based, and mixed-nutrition ( Table 8 ).
A logistic regression model was performed with impaired Conners scales as the dependent variable and the study groups as independent variables, controlled for gender, maternal education, and age at examination. The model used first the exposed group and then the control group as the reference group for the different exposure statuses. Following adjustment, the differences between the exposed and the control groups remained significant, and the same trend was evident for the soy-based groups, although not statistically significant ( Table 9 ).
Correlations Among the SB, Sensory Profile, and Conners Scores
The correlation coefficients between the sensory profile score and scores of selected SB subtests were statistically significant, but very low (0.15–0.19). The same low but significant correlation coefficients were seen between the scores on the Conners rating scales and selected SB subtests (0.15–0.22). Because a Conners rating scale score below normal may be associated with a low WM score, we studied this point: 12.5% of children with a WM score ≥90 exhibited a Conners score below normal as compared with 25.6% of children with a WM score <90 (P value = 0.027).
The Spearman correlation coefficient between the sensory profile score and the Conners rating scales was 0.497 and was statistically significant (P < 0.001).
Characterization of the Most Impaired Children
Very few children (4,5,6,7,8) were found to have scores indicating a problem on all three examinations, SB, sensory profile, and Conners rating scales. The most impaired children were distributed across all the soy-based formula groups and not exclusively in the exposed group.
Discussion
In this study, we evaluated the development of children who were exposed to a diet based exclusively on a vitamin B1-deficient soy-based formula during their first year and hence might have suffered subclinical thiamine deficiency. There was only a slight, usually statistically insignificant reduction in several parameters of the exposed children’s cognitive abilities on the SB subtests. Their verbal abilities and QR were lower than those of the controls, and the proportion of children scoring <90 on several subtests was higher, but most of these differences disappeared following adjustment for well-known confounders. All children who were fed soy-based formulas had a higher score on the Conners questionnaire, implying a higher risk of ADHD and an impaired sensory profile, but there were no differences between those who were fed the B1-deficient or B1-adequate soy-based formulas.
Outcomes of children affected by the B1-deficient diet in Israel have been reported in several studies (22,23,24,25). Fattal-Valevski et al. (22). described nine children hospitalized in their medical center because of severe thiamine deficiency; most of them had severe gastrointestinal symptoms, failure to thrive, and behavioral and neurological changes, including ophthalmoplegia and nystagmus in three cases. Parenteral treatment with high doses of thiamine brought marked improvement of the clinical symptoms. On the basis of the medical records of seven of the children, these authors (23) found that epilepsy may also be a late outcome of severe B1 deficiency in infancy. The same authors (24) reported on outcomes in children exhibiting milder thiamine deficiency: those fed the B1-deficient formula who did not present with acute signs of beriberi at the time of exposure but who might have had nonspecific gastrointestinal symptoms. When these children were compared with 20 control children from the outpatient pediatric clinic, the former exhibited a reduction in expressive language abilities and auditory comprehension of language. The authors noted a possible selection bias in this study stemming from the hospital-based setting. Fattal-Valevski et al. (25). recently published another report, this one focusing on the development of syntax and lexical retrieval in children who consumed a B1-deficient formula—not necessarily exclusively—for at least four consecutive weeks under the age of 13 mo. They found that 57 (97%) of the 59 exposed children had syntactic and lexical deficits as compared with 9% of the controls, implying a mechanism that is “all-or-none” rather than dose responsive.
Our results differ from those of Fattal-Valevski et al., perhaps because of different methodologies and different outcomes measured. Our study participants were recruited from family health centers (FHCs) all over Israel, making them population based and lowering the potential for a selection bias. Our children had no distinct clinical signs of vitamin B1 deficiency, and none of them had been hospitalized. Exposure data in our survey were based on the FHC files, which were updated before the Remedia event, decreasing the potential for an information bias. We were able to adjust our results for strong confounders such as gender, maternal education, and maternal smoking, which apparently explained a large proportion of the differences found. Finally, our examination team was blinded to the exposure status of the child, avoiding a potential observer bias, whereas this was not the case in the studies by Fattal-Valevski et al. Despite these differences in methodology, when the Bayley scales of the exposed children in the study by Fattal-Valevski et al. (23) used to assess their motor, language, and cognitive development were controlled for verbal abilities, they were similar to those of the controls; thus, our findings that the full score IQ, verbal IQ, and nonverbal IQ scores were not significantly different between the exposed and the control children do not completely contradict the findings by Fattal-Valevski and colleagues (23).
A higher number of our exposed children presented with scores <90 in various SB subtests. This was also true when the exposed group was compared with all control groups combined. There were no differences in the mean scores calculated for the strata of children with scores of 90 and above, implying that children with scores <90 might have been slightly affected whereas the others were not. Additional evidence in this direction is the inverse correlation found between the length of exposure to the B1-deficient diet and the SB scores. However, other factors seem to have a strong impact as well (e.g., gender, maternal education), and controlling for them often resulted in dilution of the differences noted, implying a combined effect of the exposure and personal and environmental elements.
The correlations between the scores of selected SB subtests and the sensory profile score and Conners rating scales scores were low but statistically significant, and in line with our previous findings of a significant association between sensory deficits, ADHD, and reduced intellectual abilities (26).
The Conners scales and the sensory profile results showed a significant difference between the control group and all groups fed nondairy formulas, but no distinct difference between the exposed and nonexposed children. In other words, children who were fed soy formulas were more likely to have ADHD and sensory modulation symptoms than children who were breast-fed or consumed other milk substitutes, as evidenced by the Conners scale scores and the sensory profile results. This finding supports the speculation that some of the babies may have been switched to soy formulas because of excessive crying, feeding problems, and being “difficult to handle” in general, all symptoms associated with regulatory disorders, e.g., reversed causality. This hypothesis gained support from the finding that mothers of children in the groups of exposed and mixed nutrition were more likely to report fetal distress than mothers of children in the control group. We found that the two main reasons for the change to nondairy formulas were intolerance to cow’s milk and self-decision. A physician’s recommendation was less frequently reported as the reason for the change.
Infants with regulatory disorders, which can interfere with normal growth and behavior, have a higher rate of behavioral–emotional disorders in childhood. A meta-analysis of 22 studies by Hemmi et al. (27) explored the possible association between early infant regulatory problems and later childhood behavioral problems, including ADHD; they found a higher rate of behavioral problems among the children with early regulatory disturbances as compared with controls. Hence, our finding of a higher rate of impaired Conners scales and sensory profile results in all children exposed to soy-based milk regardless of its B1 content is in line with these studies.
Thiamine deficiency is known to affect the brain, and certainly, the rapidly developing brain of the young child. Several mechanisms are suggested, one of which is increased oxidative stress and early microglia (brain macrophages) activation (28). The brain structures most affected by thiamine deficiency are the thalamic nuclei, several other nuclei, and the mammillary bodies; all of them share high thiamine turnover and high rates of oxidative metabolism (10). These are also the areas where magnetic resonance imaging changes are observed in patients with thiamine deficiency. Todd and Butterworth (4) found that microglia activation occurs around 8 d following the initiation of acute thiamine deficiency in rats, and precedes the damage to the blood–brain barrier by 2 d. Microglial cells produce reactive oxygen species and nitric oxide, thereby increasing oxidative stress that may promote neuronal death (28).
The main strengths of the current study are its size, the nonselective character of the sample, and the high participation rate of the study population. Another advantage is the fact that exposure data were based on FHC notes recorded before the outbreak of the Remedia event, making them less prone to misclassification and information bias. In addition, information on strong confounders such as maternal education was available and enabled adjustment of the results. It must be pointed out, however, that when dietary data in the FHC records were limited or unavailable, we had to count on retrospective parental reporting, which may have been distorted by time. Having only parental input for the Conners scales and not the teacher’s assessment may also be a limiting factor.
In conclusion, the results of our study do not support an association between subclinical vitamin B1 deficiency in infancy and long-term effects. The association between soy-based formulas, regardless of their B1 content, and impaired sensory profile and ADD/ADHD suggest reversed causality.
Methods
Study Design and Population
This was a historical prospective cohort study.
Sampling frame. The study cohort was drawn from the databases of the national and health maintenance organizations’ FHCs. The FHCs were established almost 100 y ago to provide immunizations, early detection, and education for parents about babies’ nutrition, growth, and development (29). Today, some 1,300 FHCs in Israel offer preventive services and growth and development follow-up for most newborns in the country. Details on babies’ nutrition are routinely recorded in the FHC files.
Inclusion criteria for the study consisted of being Jewish and born between 1 October 2002 and 7 October 2003. Exclusion criteria included being Arab (because the assessment tools used in this survey are validated for Hebrew-speaking children only), born premature (in the 33rd week of pregnancy or before), low birth weight (below 1,800 g), an IQ score <70 on the SB intelligence scale, or specific, clinically diagnosable developmental disorders (such as Down syndrome).
All relevant files in FHCs were screened for exposure status. “Exposure” was defined as having been fed the thiamine-deficient formula exclusively for at least four consecutive weeks in the first year of life, between 1 May and 7 November 2003, based on the 1-mo average in which B1 is stored in the body. Nonexposure was defined as being fed the B1-deficient formula concomitantly with breastfeeding, other formulas solid foods or fed the B1-deficient formula for <4 wk; being breast-fed exclusively or being fed dairy-based formulas; being fed brands of soy-based formulas other than Remedia during the same time period.
For each exposed baby identified, one or two nonexposed infants from the same FHC whose files were consecutive to the file of the exposed infant were selected.
The parents of all exposed and nonexposed children were contacted by phone and asked to participate in the study. Those who agreed were invited for an examination, during which they also signed an informed consent form. The institutional review board of the Hadassah Medical Center approved the study.
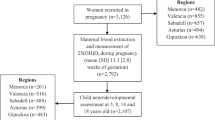
Study sample. The cohort was divided into four groups: (i) the exposed group (n = 216), and three control groups: (ii) the control group, babies who were breast-fed or consumed dairy-based formulas (n = 101); (iii) the mixed-nutrition group, babies fed the thiamine-deficient formula concomitantly with other foods (n = 71); and (iv) the soy-based group, babies fed non “Remedia” soy-based formulas (N = 42).
Of the 662 children identified in the databases of the FHCs, 290 were defined as exposed. A total of 66 children did not meet the inclusion criteria, and the parents of another 166 children declined to participate in the study (74, 50, 31, and 12 in the exposed, control, soy-based, and mixed-nutrition groups, respectively). Thus, 430 children were enrolled in the study. The response rate of those eligible (662 −66 = 596) was 75.5% (430/596), or 74.5%, 66.2%, 58.3%, and 86.7% in the exposed, control, soy-based, and mixed-nutrition groups, respectively.
Study Tools
Each participant was invited for a comprehensive medical, neurodevelopmental, and psychological examination. The evaluation was conducted by a team composed of a physician and a psychologist, both blinded to the group definition of the child being examined, and a registrar.
Physical assessment. A pediatrician experienced in child development assessed the child’s physical and neurodevelopmental status according to a specially designed questionnaire on pregnancy and delivery and a medical and neurological evaluation form used routinely for research purposes in the Jerusalem Child Development Center (13,14); major and minor anomalies and motor activity according to a table of age-adjusted motor development milestones for ages 3, 3½, 4, and 5 y were recorded.
Cognitive development assessment. A developmental psychologist administered the SB Intelligence Scale, 5th edition (30). The SB scale is composed of five subtests: WM, fluid reasoning, knowledge, QR, and visual-spatial processing. All factors are assessed in verbal and nonverbal domains. A global, FSIQ score is provided in addition to VIQ, NVIQ, and five composite factor scores, all based on a mean of 100 and a SD of 15. In accordance with the study’s exclusion criteria, children with an FSIQ score of 70 or below were excluded.
Sensory profile (31,32). The sensory profile is a standardized questionnaire appropriate for children aged 3–10 y. It assesses sensory modulation and the degree, intensity, and nature of the responses to sensory input. Parents complete the questionnaire by reporting how frequently their children respond in the way described by each item, using a 5-point Likert scale (nearly never, seldom, occasionally, frequently, and almost always). Low scores reflect undesirable behaviors. Total scores were also obtained from the short sensory profile, a standardized, abbreviated version of the sensory profile. Reliability includes internal consistency for the various sections that ranged from 0.47 to 0.91.
The Conners rating scales—revised. The Conners rating scales—revised (CRS-R (33,34)) assess behavioral, cognitive, familial, anger control, anxiety, and social problems as well as academic and emotional behavior in children aged 3–17 y. This screening tool is sensitive to a range of syndromes ranging from inattention to “hyperactivity” (ADD and ADHD). The questionnaire has two forms, one filled out by the parent and one by the teacher.
Questionnaires for parents. Parents were asked to self-report on their level of education, current smoking and smoking during pregnancy, and medical status, and on their child’s prenatal, neonatal, and postnatal history; feeding; and medical history.
Study Variables
Demographic variables. Parent’s level of education was used as a continuous and a discrete (less than or equal to/more than 12 y) variable. Socioeconomic status was estimated by the Israel Central Bureau of Statistics index adjusted for the residence address. This index ranges from 0 to 20, where 0 represents the lowest and 20 the highest socioeconomic status code. The socioeconomic status variable was used either as a continuous variable or as a discrete one (by three categories: 0–10, 11–14, and 15–20).
Prenatal, neonatal, and postnatal variables. Gestational age (in weeks), complications at labor (yes/no), birth weight (in grams), birth week, and fetal distress (yes/no) were obtained from the FHC files and the parents questionnaires.
Motor, sensory, and cognitive tests. The motor milestones were summarized according to age-related activities expected to be performed by the child. Performance was considered impaired when <90% of the activities were performed. Walking-initiation age (younger/older than 18 mo, based on accepted norms) was used to rule out the possibility that the child with a normal motor test at age 5 y was actually slow in development.
The mean scores (and SDs) of the study groups on the SB subtests were compared among groups. A discrete variable denoting scores <90 or ≥90 was developed; this cutoff point, which is about 1 SD below the mean score of our control group, was chosen because it is an acceptable reference for “normal” vs. “subnormal” scores.
The sensory profile (35) establishes scores based on SD from the normative data. Scores 1–2 SDs below the mean fall into the “Probable Difference” category; scores >2 SDs below the mean are in the “Definite Difference” category. As stated in the manual (35), scores in these two categories indicate atypical behaviors associated with sensory processing problems. In addition, a total score on the short sensory profile was recorded as a composite binary outcome of “a problem” vs. “no problem,” and the frequency of the outcome “a problem” was compared across the different study groups.
Only the questionnaires filled out by parents were used for the Conners rating scales due to low compliance of the teachers. A score above 60 suggested a clinical problem.
To better define the most impaired children, a composite binary outcome of “any problem” (SB-FSIQ or VIQ or NVIQ or QR score <90; a sensory profile score suggestive of a problem; or Conners rating scales above 60) vs. “no problem” was created.
Data Analysis
The study groups were compared using the least squares procedure to fit general linear models with Scheffe correction for continuous variables: age at test, gestational age, birth weight, birth week, maternal education, and socioeconomic status index, as detailed above, using the exposed group as the reference group. χ2 test was performed for discrete variables, including current maternal smoking (known to affect SB scores).
Means of the SB individual subtests were compared between the groups, and univariate and multivariate linear regression models were used to study the independent effect of the B1-deficient formula, controlled for potential confounders (gender, maternal education, age at the test), on the test scores. In addition, the study groups were compared by the proportion of children who scored <90 on each of the SB subtests and univariate and multivariate logistic regression models were used to study the independent effect of the B1-deficient formula, controlled for potential confounders on the SB results (<90/≥90).
The potential impact of age at exposure and exposure duration on SB scores <90 in the exposed group was examined by applying logistic regression models.
Logistic regression models were used to evaluate the relationships between the exposure status and the sensory profile score.
Groups were compared by the proportion of children with a score >60 on the ADHD index, and multiple logistic regression models were applied to study the independent effect of the B1-deficient formula, controlled for potential confounders.
Correlations between selected SB subtests, the sensory profile score, and the Conners rating scales were examined by the Spearman correlation coefficient. This analysis was also used to evaluate the correlation between the sensory profile and the SB scores.
The composite binary outcome of “any problem” vs. “no problem” was compared across the different study groups.
All analyses were done with the SAS statistical program, version 9.1.3 (SAS Institute, Cary, NC). Statistical significance was set at <0.05.
Two other subanalyses were performed: (i) stratifying by age at examination (3–4 y and 5–6 y), which yielded statistically insignificant results; and (ii) comparing the exposed group with the three control groups combined, in order to increase the study power.
Statement of Financial Support
This work was supported by grant 0394142 from the Israeli Ministry of Health.
References
Davis RE, Icke GC . Clinical chemistry of thiamine. Adv Clin Chem 1983;232:93–140.
Food and Nutrition Board, Institute of Medicine. Thiamine. Dietary Reference Intakes: Thiamine, Riboflavin, Niacin, Vitamin B-6, Vitamin B-12, Pantothenic Acid, Biotin, and Choline. Washington DC: National Academy Press, 1998: 58–86.
WHO Publication WHO/NHD/99.13. Thiamine deficiency and its prevention and control in major emergencies, 1999. (http://www.who.int/nut/documents/thiamine_in_emergencies_eng.pdf).
Todd K, Butterworth RF . Mechanisms of selective neuronal cell death due to thiamine deficiency. Ann N Y Acad Sci 1999;893:404–11.
Kliegman RM, Behrman RE, Jenson HB, Stanton BF . Nelson Textbook of Pediatrics, 18th edn. Philadelphia, PA: Saunders Elsevier, 2007: 246–7.
Cook CC, Hallwood PM, Thomson AD . B Vitamin deficiency and neuropsychiatric syndromes in alcohol misuse. Alcohol Alcohol 1998;33:317–36.
Bender DA . Optimum nutrition: thiamin, biotin and pantothenate. Proc Nutr Soc 1999;58:427–33.
Tanphaichitr V . Thiamine. In: Shils M, ed. Nutrition in Health and Disease. 9th edn. Baltimore: Williams & Wilkins, 1999: 381–9.
Müri RM, Von Overbeck J, Furrer J, Ballmer PE . Thiamin deficiency in HIV-positive patients: evaluation by erythrocyte transketolase activity and thiamin pyrophosphate effect. Clin Nutr 1999;18:375–8.
Zuccoli G, Gallucci M, Capellades J, et al. Wernicke encephalopathy: MR findings at clinical presentation in twenty-six alcoholic and nonalcoholic patients. AJNR Am J Neuroradiol 2007;28:1328–31.
Sebrell WH Jr . IV. Clinical nutrition in the United States. Am J Public Health Nations Health 1968;58:2035–42.
Ivanovic DM, Leiva BP, Pérez HT, et al. Head size and intelligence, learning, nutritional status and brain development. Head, IQ, learning, nutrition and brain. Neuropsychologia 2004;42:1118–31.
Ornoy A, Michailevskaya V, Lukashov I, Bar-Hamburger R, Harel S . The developmental outcome of children born to heroin-dependent mothers, raised at home or adopted. Child Abuse Negl 1996;20:385–96.
Ornoy A, Segal J, Bar-Hamburger R, Greenbaum C . Developmental outcome of school-age children born to mothers with heroin dependency: importance of environmental factors. Dev Med Child Neurol 2001;43:668–75.
Ornoy A . The effects of alcohol and illicit drugs on the human embryo and fetus. Isr J Psychiatry Relat Sci 2002;39:120–32.
Ornoy A . The impact of intrauterine exposure versus postnatal environment in neurodevelopmental toxicity: long-term neurobehavioral studies in children at risk for developmental disorders. Toxicol Lett 2003;140-141:171–81.
Ivanovic DM, Olivares MG, Castro CG, Ivanovic RM . Nutrition and learning in Chilean school age children: Chile’s Metropolitan Region Survey 1986-1987. Nutrition 1996;12:321–8.
Ivanovic DM, Leiva BP, Perez HT, et al. Long-term effects of severe undernutrition during the first year of life on brain development and learning in Chilean high-school graduates. Nutrition 2000;16:1056–63.
Grantham-McGregor SM, Fernald LC . Nutritional deficiencies and subsequent effects on mental and behavioral development in children. Southeast Asian J Trop Med Public Health 1997;28: Suppl 2:50–68.
Bryan J, Osendarp S, Hughes D, Calvaresi E, Baghurst K, van Klinken JW . Nutrients for cognitive development in school-aged children. Nutr Rev 2004;62:295–306.
Vikhanski L . Fatal flaw in baby formula sparks reform in Israeli ministry. Nat Med 2004;10:7.
Fattal-Valevski A, Kesler A, Sela BA, et al. Outbreak of life-threatening thiamine deficiency in infants in Israel caused by a defective soy-based formula. Pediatrics 2005;115:e233–8.
Fattal-Valevski A, Bloch-Mimouni A, Kivity S, et al. Epilepsy in children with infantile thiamine deficiency. Neurology 2009;73:828–33.
Fattal-Valevski A, Azouri-Fattal I, Greenstein YJ, Guindy M, Blau A, Zelnik N . Delayed language development due to infantile thiamine deficiency. Dev Med Child Neurol 2009;51:629–34.
Fattal I, Friedmann N, Fattal-Valevski A . The crucial role of thiamine in the development of syntax and lexical retrieval: a study of infantile thiamine deficiency. Brain 2011;134(Pt 6):1720–39.
Yochman A, Parush S, Ornoy A . Responses of preschool children with and without ADHD to sensory events in daily life. Am J Occup Ther 2004;58:294–302.
Hemmi MH, Wolke D, Schneider S . Associations between problems with crying, sleeping and/or feeding in infancy and long-term behavioural outcomes in childhood: a meta-analysis. Arch Dis Child 2011;96:622–9.
Jhala SS, Hazell AS . Modeling neurodegenerative disease pathophysiology in thiamine deficiency: consequences of impaired oxidative metabolism. Neurochem Int 2011;58:248–60.
Shehory-Rubin Z, Shvartz S. “Hadassah for the health of the people”. The Health-Education Work of “Hadassah” in Eretz Israel during the British Mandate. Jerusalem, Israel: The Zionist Library 2004 (Hebrew).
Roid, GH . Stanford-Binet Intelligence Scales, 5th edn. Itasca, IL: Riverside Publishing, 2003.
Dunn W . Performance of typical children on the Sensory Profile: an item analysis. Am J Occup Ther 1994;48:967–74.
Dunn W . The sensations of everyday life: empirical, theoretical, and pragmatic considerations. Am J Occup Ther 2001;55:608–20.
Charach A, Chen S, Hogg-Johnson S, Schachar RJ . Using the Conners’ Teacher Rating Scale-Revised in school children referred for assessment. Can J Psychiatry 2009;54:232–41.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edn. (DSM IV). Washington, DC: American Psychiatric Association, 1994.
Dunn W . The Sensory Profile Manual. San Antonio, TX: The Psychological Corporation, 1999.
Acknowledgements
The authors thank Yona Amitai and Dorit Nitzan-Kaluski for their help in preparing the study proposal and Boaz Lev for assisting throughout the implementation, as well as the participating children and parents for their contribution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ornoy, A., Tekuzener, E., Braun, T. et al. Lack of severe long-term outcomes of acute, subclinical B1 deficiency in 216 children in Israel exposed in early infancy. Pediatr Res 73, 111–119 (2013). https://doi.org/10.1038/pr.2012.140
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2012.140
This article is cited by
-
A Psychometric Evaluation of the Behavioral Inhibition Questionnaire in a Non-Clinical Sample of Israeli Children and Adolescents
Journal of Child and Family Studies (2018)