Key Points
-
Chemicals that cause itch or pain in humans elicit qualitatively different behaviours in animals. Sensory responses and the electrophysiologically recorded responses to these stimuli are used to determine the populations of sensory neurons that transduce and convey the information that the brain uses to produce the sensation of itch and to distinguish itch from pain.
-
Pruritic chemicals elicit action potentials in subsets of cutaneous peripheral nociceptors that also respond with differing sensitivities to one or more types of painful stimuli. Other nociceptors are 'non-pruriceptive' and do not respond to pruritic chemicals but respond to painful capsaicin, heat or mechanical noxious stimuli.
-
In primates, two pruritic agents have been tested extensively: histamine and cowhage spicules. The afferents activated by these agents and other nociceptive afferents convey activity to the spinal dorsal horn, where they activate neurons, including spinothalamic tract (STT) neurons that provide pruriceptive and nociceptive input to the brain.
-
In the monkey, histaminergic and non-histaminergic itch are conveyed by separate subsets of nociceptive STT neurons. In both primary afferents and STT neurons, pruritic stimuli usually activate fewer nociceptive neurons and elicit lower discharge rates than moderately painful or noxious stimuli, thereby providing one means by which the brain could differentiate pruritic from noxious information.
-
In mice, diverse receptor expression and signalling pathways among subsets of pruriceptive and non-pruriceptive nociceptors have been found. Using molecular genetic tools, it was shown that selective activation of Mas-related G-protein-coupled receptor member A3 (MRGPRA3)-expressing neurons induces itch- and not pain-like behaviour, supporting the idea that specific neurons mediate itch.
-
In the dorsal horn, excitatory and inhibitory interneurons have an important role in pruriceptive transmission and several candidate neurotransmitters have been identified. Itch behaviour can be modified by several experimental manipulations and pruritogen-induced activity can be reduced by scratching.
-
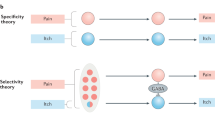
Several models have been proposed to explain how the brain might encode itch versus pain. One model suggests that itch depends on more activity in pruriceptive than non-pruriceptive nociceptors. Another model suggests that it is the spatially sparse activation of any type of cutaneous nociceptive neurons that signals itch.
-
Testing the validity of these models will be facilitated in the future by the development of methods to control and manipulate action potential activity independently in pruriceptive and non-pruriceptive neurons.
Abstract
Chemicals that are used experimentally to evoke itch elicit activity in diverse subpopulations of cutaneous pruriceptive neurons, all of which also respond to painful stimuli. However, itch is distinct from pain: it evokes different behaviours, such as scratching, and originates from the skin or certain mucosae but not from muscle, joints or viscera. New insights regarding the neurons that mediate the sensation of itch have been gained from experiments in which gene expression has been manipulated in different types of pruriceptive neurons as well as from comparisons between psychophysical measurements of itch and the neuronal discharges and other properties of peripheral and central pruriceptive neurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Chen, S. C. Pruritus. Dermatol. Clin. 30, 309–321 (2012).
Akiyama, T. & Carstens, E. Neural processing of itch. Neuroscience 250, 697–714 (2013).
Kuraishi, Y. Potential new therapeutic targets for pathological pruritus. Biol. Pharm. Bull. 36, 1228–1234 (2013).
Steinhoff, M., Cevikbas, F., Ikoma, A. & Berger, T. G. Pruritus: management algorithms and experimental therapies. Semin. Cutan. Med. Surg. 30, 127–137 (2011).
Hägermark, O., Strandberg, K. & Grönneberg, R. Effects of histamine receptor antagonists on histamine-induced responses in human skin. Acta Derm. Venereol. 59, 297–300 (1979).
Schmelz, M., Schmidt, R., Bickel, A., Handwerker, H. O. & Torebjork, H. E. Specifc C-receptors for itch in human skin. J. Neurosci. 17, 8003–8008 (1997).
Sikand, P., Shimada, S. G., Green, B. G. & LaMotte, R. H. Sensory responses to injection and punctate application of capsaicin and histamine to the skin. Pain 152, 2485–2494 (2011).
Klein, P. A. & Clark, R. A. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch. Dermatol. 135, 1522–1555 (1999).
LaMotte, R. H., Shimada, S. G., Green, B. G. & Zelterman, D. Pruritic and nociceptive sensations and dysesthesias from a spicule of cowhage. J. Neurophysiol. 101, 1430–1443 (2009).
Johanek, L. M. et al. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J. Neurosci. 27, 7490–7497 (2007).
Sikand, P., Shimada, S. G., Green, B. G. & LaMotte, R. H. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain 144, 66–75 (2009).
Sikand, P., Dong, X. & LaMotte, R. H. BAM8–22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J. Neurosci. 31, 7563–7567 (2011).
Liu, Q. et al. Mechanisms of itch evoked by β-alanine. J. Neurosci. 32, 14532–14537 (2012).
Johanek, L. M. et al. A role for polymodal C-fiber afferents in non-histaminergic itch. J. Neurosci. 28, 7659–7669 (2008).
Shimada, S. G. & LaMotte, R. H. Behavioral differentiation between itch and pain in mouse. Pain 139, 681–687 (2008).
Akiyama, T., Carstens, M. I. & Carstens, E. Differential itch- and pain-related behavioral responses and μ-opoid modulation in mice. Acta Derm. Venereol. 90, 575–581 (2010).
LaMotte, R. H., Shimada, S. G. & Sikand, P. Mouse models of acute, chemical itch and pain in humans. Exp. Dermatol. 20, 778–782 (2011).
Schmelz, M. et al. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J. Neurophysiol. 89, 2441–2448 (2003). Using microneurography to record neuronal activity from unmeylinated C-fibres in human, this paper showed that some mechano-insensitive C-fibres are pruriceptive and respond to histamine, although they also respond to a wide range of chemicals including algogens. Others are non-pruriceptive and respond to algogens but not to histamine.
Handwerker, H. O., Forster, C. & Kirchhoff, C. Discharge patterns of human C-fibers induced by itching and burning stimuli. J. Neurophysiol. 66, 307–315 (1991).
Namer, B. et al. Separate peripheral pathways for pruritus in man. J. Neurophysiol. 100, 2062–2069 (2008). Using microneurography in humans, this study found that histamine and cowhage active two separate, non-overlapping populations of unmyelinated C-fibres: that is, mechano-insensitive C-fibres and mechano-sensitive C-fibres. These findings suggest that histaminergic and non-histaminergic itch are mediated through separate pathways.
Ringkamp, M., Borzan, J., Schaefer, K., Hartke, T. V. & Meyer, R. A. Activation of polymodal nociceptors in monkey by punctate chemical stimulation with histamine and capsaicin. Soc. Neurosci. Abstr. 584.6 (2010).
Ringkamp, M. et al. A role for nociceptive, myelinated nerve fibers in itch sensation. J. Neurosci. 31, 14841–14849 (2011). The findings of this study demonstrate that activity in A-fibres is involved in mediating cowhage-induced itch and nociceptive sensations in humans and that cowhage spicules activate mechano-sensitive A-fibre nociceptors in the monkey.
Ma, C., Nie, H., Gu, Q., Sikand, P. & LaMotte, R. H. In-vivo responses of cutaneous C-mechanosensitive neurons in mouse to punctate chemical stimuli that elicit itch and nociceptive sensations in humans. J. Neurophysiol. 107, 357–363 (2011).
Schmidt, R. et al. Novel classes of responsive and unresponsive C nociceptors in human skin. J. Neurosci. 15, 333–341 (1995).
Schmelz, M., Schmidt, R., Handwerker, H. O. & Torebjörk, H. E. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain 123, 560–571 (2000).
Meyer, R. A., Davis, K. D., Cohen, R. H., Treede, R. D. & Campbell, J. N. Mechanically insensitive afferents (MIAs) in cutaneous nerves of monkey. Brain Res. 561, 252–261 (1991).
Ringkamp, M. et al. Capsaicin responses in heat-sensitive and heat-insensitive A-fiber nociceptors. J. Neurosci. 21, 4460–4468 (2001).
Lawson, J. J., McIlwrath, S. L., Woodbury, C. J., Davis, B. M. & Koerber, H. R. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. J. Pain 9, 298–308 (2008).
Han, L. et al. A subpopulation of nociceptors specifically linked to itch. Nature Neurosci. 16, 174–182 (2012). The results of this study, together with those of reference 32, demonstrate a type of primary sensory neuron that expresses receptors for multiple pruritogens and that specifically mediates itch-like behaviour in mice.
Schley, M. et al. Mechano-insensitive nociceptors are sufficient to induce histamine-induced itch. Acta Derm. Venereol. 93, 394–399 (2013).
Roberson, D. P. et al. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nature Neurosci. 16, 910–918 (2013).
Liu, Q. et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139, 135313–135365 (2009).
Liu, Q. et al. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci. Signal. 4, ra45 (2011).
Imamachi, N. et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc. Natl Acad. Sci. USA 106, 11330–11335 (2009).
Jeffry, J., Kim, S. & Chen, Z. F. Itch signaling in the nervous system. Physiology 26, 286–292 (2011).
Shim, W. S. et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J. Neurosci. 27, 2331–2337 (2007).
Wilson, S. R. et al. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nature Neurosci. 14, 595–602 (2011).
Wilson, S. R. et al. The ion channel TRPA1 is required for chronic itch. J. Neurosci. 33, 9283–9294 (2013).
Story, G. M. et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829 (2003).
Jordt, S. E. et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427, 260–265 (2004).
Bautista, D. M., Pellegrino, M. & Tsunozaki, M. TRPA1: a gatekeeper for inflammation. Annu. Rev. Physiol. 75, 181–200 (2012).
Dymecki, S. M., Ray R. S. & Kim J. C. Mapping cell fate and function using recombinase-based intersectional strategies. Methods Enzymol. 477, 183–213 (2010).
Reddy, V. B., Iuga, A. O., Shimada, S. G., LaMotte, R. H. & Lerner, E. A. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease activated receptors. J. Neurosci. 28, 4331–4335 (2008).
Wilson, S. R. et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2, 285–295 (2013).
Ringkamp, M. et al. A subclass of cutaneous polymodal nociceptive C fiber afferents in non human primates responds to β-alanine. Soc. Neurosci. Abstr. 556.07 (2013).
Zylka, M. J., Rice, F. L. & Anderson, D. J. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron 45, 17–25 (2005).
Rau, K. K. et al. Mrgprd enhances excitability in specific populations of cutaneous murine polymodal nociceptors. J. Neurosci. 29, 8612–8619 (2009).
Cavanaugh, D. J. et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc. Natl Acad. Sci. USA 106, 9075–9080 (2009).
Bickford, R. G. Experiments relating to the itch sensation, its peripheral mechanism and central pathways. Clin. Sci. 3, 377–386 (1938).
Hyndman, O. R. & Wolkin, J. Anterior cordotomy: further observations on the physiologic results and optimum manner of performance. Arch. Neuro. Psychiatry 50, 129–148 (1943).
Simone, D. A. et al. Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J. Neurophysiol. 91, 213–222 (2004).
Davidson, S. et al. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J. Neurosci. 27, 10007–10014 (2007).
Davidson, S. et al. Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J. Neurophysiol. 108, 1711–1723 (2012). With the use of antidromic stimulation at the thalamus to identify spinothalamic dorsal horn neurons in monkeys, this study, together with reference 52, found that separate populations of nociceptive dorsal horn neurons in primate are activated by histamine and cowhage spicules.
Atanassoff, P. G. et al. Enhancement of experimental pruritus and mechanically evoked dysesthesiae with local anesthesia. Somatosens. Mot. Res. 16, 291–298 (1999).
Todd, A. J. Neuronal circuitry for pain processing in the dorsal horn. Nature Rev. Neurosci. 11, 823–836 (2010).
Xu, Y. et al. Ontogeny of excitatory spinal neurons processing distinct somatic sensory modalities. J. Neurosci. 33, 14738–14748 (2013).
Wang, X. et al. Excitatory superficial dorsal horn interneurons are functionally heterogeneous and required for the full behavioral expression of pain and itch. Neuron 78, 312–324 (2013).
Sun, Y. G. & Chen, Z. F. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448, 700–703 (2007).
Sun, Y. G. et al. Cellular basis of itch sensation. Science 325, 1531–1534 (2009). These findings, along with those of reference 58, support the conclusion that neurons in the dorsal horn that express the receptor for GRP are necessary for itch-like behaviour in the mouse.
Mishra, S. K. & Hoon M. A. The cells and circuitry for itch responses in mice. Science 340, 968–971 (2013).
Zhang, F. X. et al. Inhibition of inflammatory pain by activating B-type natriuretic peptide signal pathway in nociceptive sensory neurons. J. Neurosci. 30, 10927–10938 (2010).
Akiyama, T., Tominaga, M., Takamori, K., Carstens, M. I. & Carstens, E. Roles of glutamate, substance P, and gastrin-releasing peptide as spinal neurotransmitters of histaminergic and nonhistaminergic itch. Pain http://dx.doi.org/10.1016/j.pain.2013.09.011 (2013).
Lagerstrom, M. C. et al. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron 68, 529–542 (2010).
Liu, Y. et al. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron 68, 543–556 (2010). The findings of this study, together with reference 63, support the conclusion that neurons expressing VGLUT2 exert an inhibitory action on itch transmission in the mouse. Mice lacking VGLUT2 in most nociceptors exhibit enhanced itch-like behaviour and exhibit itch- rather than pain-like behaviour in response to capsaicin injection.
Ross, S. E. et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron 65, 886–898 (2010). These authors discovered that a specific population of inhibitory interneurons in the spinal cord acts to suppress itch-like behaviour in the mouse; the loss of these neurons results in a pathological increase in itch.
Ross, S. E. Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Curr. Opin. Neurobiol. 21, 880–887 (2011).
Kam, P. C. & Tan, K. H. Pruritus — itching for a cause and relief? Anaesthesia 51, 1133–1138 (1996).
Ko, M. C. & Naughton, N. N. An experimental itch model in monkeys: characterization of intrathecal morphine-induced scratching and antinociception. Anesthesiology 92, 795–805 (2000).
Moser, H. R. & Giesler, G. J. Jr. Itch and analgesia resulting from intrathecal application of morphine: contrasting effects on different populations of trigeminothalamic tract neurons. J. Neurosci. 33, 6093–6101 (2013).
Liu, X. Y. et al. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell 147, 447–458 (2011).
LaMotte, R. H., Shain, C. N., Simone, D. A. & Tsai, E. F. P. Neurogenic hyperalgesia psychophysical studies of underlying mechanisms. J. Neurophysiol. 66, 190–211 (1991).
Ikoma, A. et al. Painful stimuli evoke itch in patients with chronic pruritus: central sensitization for itch. Neurology 62, 212–217 (2004).
Simone, D. A. et al. Neurogenic hyperalgesia: central neural correlates in responses of spinothalamic tract neurons. J. Neurophysiol. 66, 228–246 (1991).
Sandkühler, J. Models and mechanisms of hyperalgesia and allodynia. Physiol. Rev. 89, 707–758 (2009).
Baron, R., Schwarz, K., Kleinert, A., Schattschneider, J. & Wasner, G. Histamine induced itch converts into pain in neuropathic hyperalgesia. Neuroreport 12, 3475–3478 (2001).
Brull, S. J., Atanassoff, P. G., Silverman, D. G., Zhang, J. & LaMotte, R. H. Attenuation of experimental pruritus and mechanically evoked dysesthesiae in an area of cutaneous allodynia. Somatosens. Mot. Res. 16, 299–303 (1999).
Yosipovitch, G., Fast, K. & Bernhard, J. D. Noxious heat and scratching decrease histamine-induced itch and skin blood flow. J. Invest. Dermatol. 125, 1268–1272 (2005).
Davidson, S., Zhang, X., Khasabov, S. G., Simone, D. A. & Giesler, G. J. Jr. Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract. Nature Neurosci. 12, 544–546 (2009).
Akiyama, T. Iodi Carstens, M. & Carstens, E. Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PLoS ONE 6, e22665 (2011).
Ma, Q. Population coding of somatic sensations. Neurosci. Bull. 28, 91–99 (2012).
McMahon, S. B. & Koltzenburg, M. Itching for an explanation. Trends Neurosci. 15, 497–501 (1992).
Ikoma, A., Handwerker, H., Miyachi, Y. & Schmelz, M. Electrically evoked itch in humans. Pain 113, 148–154 (2005).
Tuckett, R. P. Itch evoked by electrical stimulation of the skin. J. Invest. Dermatol. 79, 368–373 (1982).
Namer, B. & Reeh, P. Scratching an itch. Nature Neurosci. 16, 117–118 (2013).
Koppert, W., Reeh, P. W. & Handwerker, H. O. Conditioning of histamine by bradykinin alters responses of rat nociceptors and human itch sensation Neurosci. Lett. 152, 117–120 (1993).
Finger, S. & Wade, N. J. The neuroscience of Helmholtz and the theories of Johannes Müller. Part 2: sensation and perception. J. Hist. Neurosci. 11, 234–254 (2002).
Acknowledgements
The authors are supported in part by US National Institutes of Health grants P01 NS47399 P01 NS 047399 (R.H.L. and M.R.) and GM087369 and NS054791 (X.D.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Noxious stimuli
-
Stimuli that are overtly or potentially damaging to normal tissues or are normally associated with pain-like (nociceptive) sensations, such as pricking, stinging or burning, that are unpleasant but may or may not hurt.
- Nociceptors
-
High-threshold peripheral receptors or sensory neurons that transduce and encode noxious stimuli.
- Pruriceptors
-
Nociceptors that respond to one or more pruritic chemicals.
- Cutaneous receptive fields
-
Areas of skin within which a stimulus activates a sensory neuron, for example, by evoking action potentials.
- Central sensitization
-
An enhanced responsiveness of nociceptive neurons in the CNS to normal input from peripheral sensory neurons.
- 'Labelled line' pathway
-
A pathway serving a particular sensory quality, such as itch. When selectively activated, it will elicit that type of sensation regardless of the type of activating stimulus.
Rights and permissions
About this article
Cite this article
LaMotte, R., Dong, X. & Ringkamp, M. Sensory neurons and circuits mediating itch. Nat Rev Neurosci 15, 19–31 (2014). https://doi.org/10.1038/nrn3641
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3641
This article is cited by
-
Cutaneous Components Leading to Pruritus, Pain, and Neurosensitivity in Atopic Dermatitis: A Narrative Review
Dermatology and Therapy (2024)
-
Peripheral signaling pathways contributing to non-histaminergic itch in humans
Journal of Translational Medicine (2023)
-
Glutamatergic Neurons in the Zona Incerta Modulate Pain and Itch Behaviors in Mice
Molecular Neurobiology (2023)
-
Neuronal FcεRIα directly mediates ocular itch via IgE-immune complex in a mouse model of allergic conjunctivitis
Journal of Neuroinflammation (2022)
-
Exploring neuronal mechanisms involved in the scratching behavior of a mouse model of allergic contact dermatitis by transcriptomics
Cellular & Molecular Biology Letters (2022)