Key Points
-
Vision is limited by spatial resolution, which is determined by photoreceptor spacing in the retina as well as the size, spacing and number of receptive fields (RFs) along the visual pathway.
-
To overcome this limitation, we can either 'overtly' attend to select objects of interest by moving our eyes to focus them on the part of the retina with highest spatial resolution — the fovea — or we can 'covertly' shift spatial attention without moving the eyes.
-
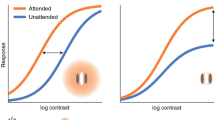
Covert spatial attention can enhance spatial resolution in various behavioural tasks, such as visual search, acuity tasks and texture segmentation tasks, at the cost of resolution at unattended locations. Moreover, covert attention distorts the perception of spatial features, such as spatial frequency, size and shape of objects as well as the distance between them.
-
Physiologically, attention shifts cortical RFs towards the focus of attention. Furthermore, RFs shrink when attention is directed to their location and RFs expand towards the focus of attention when it is directed nearby. These changes can qualitatively explain most of the behavioural findings.
-
The shifts of RFs selectively concentrate processing resources at the attentional focus and thereby enhance the representation of attended stimuli compared with unattended stimuli, which could underlie improved performance with attention in tasks limited by spatial resolution. RF shifts can distort the perception of space under the assumption of a labelled-line code for spatial position by changing the position of the RFs without updating their label.
-
RF shrinkage can improve spatial resolution performance by reducing spatial integration, thus excluding distracting information. RF shrinkage can modulate texture segmentation performance by changing the match between the texture scale and filter size.
-
To make a quantitative link between physiological and behavioural findings, future research would benefit from using common or more comparable paradigms.
Abstract
Attention allows us to select relevant sensory information for preferential processing. Behaviourally, it improves performance in various visual tasks. One prominent effect of attention is the modulation of performance in tasks that involve the visual system's spatial resolution. Physiologically, attention modulates neuronal responses and alters the profile and position of receptive fields near the attended location. Here, we develop a hypothesis linking the behavioural and electrophysiological evidence. The proposed framework seeks to explain how these receptive field changes enhance the visual system's effective spatial resolution and how the same mechanisms may also underlie attentional effects on the representation of spatial information.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Levi, D. M., Klein, S. A. & Aitsebaomo, A. P. Vernier acuity, crowding and cortical magnification. Vision Res. 25, 963–977 (1985).
Martin, G. Psychophysics. Limits of visual resolution. Nature 319, 540 (1986).
Kitterle, F. L. Psychophysics of lateral tachistoscopic presentation. Brain Cogn. 5, 131–162 (1986).
Rovamo, J., Virsu, V. & Nasanen, R. Cortical magnification factor predicts the photopic contrast sensitivity of peripheral vision. Nature 271, 54–56 (1978).
Wright, M. J. & Johnston, A. Spatiotemporal contrast sensitivity and visual field locus. Vision Res. 23, 983–989 (1983).
Nakayama, K. & Mackeben, M. Sustained and transient components of focal visual attention. Vision Res. 29, 1631–1647 (1989).
Yeshurun, Y. & Carrasco, M. Attention improves or impairs visual performance by enhancing spatial resolution. Nature 396, 72–75 (1998). This study provides a strong link between attention and spatial resolution by using a texture segmentation task in which the higher resolution brought about by attention impairs performance.
Yeshurun, Y. & Carrasco, M. Spatial attention improves performance in spatial resolution tasks. Vision Res. 39, 293–306 (1999).
Posner, M. I. Orienting of attention. Q. J. Exp. Psychol. 32, 3–25 (1980).
Liu, T., Stevens, S. T. & Carrasco, M. Comparing the time course and efficacy of spatial and feature-based attention. Vision Res. 47, 108–113 (2007).
Hawkins, H. L. et al. Visual attention modulates signal detectability. J. Exp. Psychol. Hum. Percept. Perform. 16, 802–811 (1990).
Lu, Z. L. & Dosher, B. A. External noise distinguishes attention mechanisms. Vision Res. 38, 1183–1198 (1998).
Carrasco, M., Penpeci-Talgar, C. & Eckstein, M. Spatial covert attention increases contrast sensitivity across the CSF: support for signal enhancement. Vision Res. 40, 1203–1215 (2000).
Ling, S. & Carrasco, M. Sustained and transient covert attention enhance the signal via different contrast response functions. Vision Res. 46, 1210–1220 (2006).
Herrmann, K., Montaser-Kouhsari, L., Carrasco, M. & Heeger, D. J. When size matters: attention affects performance by contrast or response gain. Nature Neurosci. 13, 1554–1559 (2010).
Carrasco, M. & McElree, B. Covert attention accelerates the rate of visual information processing. Proc. Natl Acad. Sci. USA 98, 5363–5367 (2001).
Carrasco, M., Giordano, A. M. & McElree, B. Attention speeds processing across eccentricity: feature and conjunction searches. Vision Res. 46, 2028–2040 (2006).
Carrasco, M., Giordano, A. M. & McElree, B. Temporal performance fields: visual and attentional factors. Vision Res. 44, 1351–1365 (2004).
Scholte, H. S. Spekreijse, H. & Roelfsema, P. R. The spatial profile of visual attention in mental curve tracing. Vision Res. 41, 2569–2580 (2001).
Carrasco, M. Visual attention: the past 25 years. Vision Res. 51, 1484–1525 (2011).
Rolfs, M. & Carrasco, M. Rapid simultaneous enhancement of visual sensitivity and perceived contrast during saccade preparation. J. Neurosci. 32, 13744–13752a (2012).
Zhao, M., Gersch, T. M., Schnitzer, B. S., Dosher, B. A. & Kowler, E. Eye movements and attention: the role of pre-saccadic shifts of attention in perception, memory and the control of saccades. Vision Res. 74, 40–60 (2012).
Lennie, P. The cost of cortical computation. Curr. Biol. 13, 493–497 (2003).
Cheal, M. & Lyon, D. R. Central and peripheral precuing of forced-choice discrimination. Q. J. Exp. Psychol. A 43, 859–880 (1991).
Muller, H. J. & Rabbitt, P. M. Reflexive and voluntary orienting of visual attention: time course of activation and resistance to interruption. J. Exp. Psychol. Hum. Percept. Perform. 15, 315–330 (1989).
Busse, L., Katzner, S. & Treue, S. Temporal dynamics of neuronal modulation during exogenous and endogenous shifts of visual attention in macaque area MT. Proc. Natl Acad. Sci. USA 105, 16380–16385 (2008).
Giordano, A. M., McElree, B. & Carrasco, M. On the automaticity and flexibility of covert attention: a speed–accuracy trade-off analysis. J. Vis. 9, 30 (2009).
Cameron, E. L., Tai, J. C., Eckstein, M. P. & Carrasco, M. Signal detection theory applied to three visual search tasks — identification, yes/no detection and localization. Spat. Vis. 17, 295–325 (2004).
Montagna, B., Pestilli, F. & Carrasco, M. Attention trades off spatial acuity. Vision Res. 49, 735–745 (2009).
Talgar, C. P., Pelli, D. G. & Carrasco, M. Covert attention enhances letter identification without affecting channel tuning. J. Vis. 4, 22–31 (2004).
Morgan, M. J., Ward, R. M. & Castet, E. Visual search for a tilted target: tests of spatial uncertainty models. Q. J. Exp. Psychol. A 51, 347–370 (1998).
Baldassi, S. & Burr, D. C. Feature-based integration of orientation signals in visual search. Vision Res. 40, 1293–1300 (2000).
Morrone, M. C., Denti, V. & Spinelli, D. Different attentional resources modulate the gain mechanisms for color and luminance contrast. Vision Res. 44, 1389–1401 (2004).
Pestilli, F., Viera, G. & Carrasco, M. How do attention and adaptation affect contrast sensitivity? J. Vis. 7, 9 (2007).
Pestilli, F. & Carrasco, M. Attention enhances contrast sensitivity at cued and impairs it at uncued locations. Vision Res. 45, 1867–1875 (2005).
Luck, S. J. et al. Effects of spatial cuing on luminance detectability: psychophysical and electrophysiological evidence for early selection. J. Exp. Psychol. Hum. Percept. Perform. 20, 887–904 (1994).
Ling, S. & Carrasco, M. When sustained attention impairs perception. Nature Neurosci. 9, 1243–1245 (2006).
Barbot, A., Landy, M. S. & Carrasco, M. Exogenous attention enhances 2nd-order contrast sensitivity. Vision Res. 51, 1086–1098 (2011).
Moran, J. & Desimone, R. Selective attention gates visual processing in the extrastriate cortex. Science 229, 782–784 (1985). On the basis of the finding that attention biases the response to two stimuli inside a neuron's RF in favour of the attended stimulus, the authors are the first to suggest that attention alters spatial integration by contracting RFs around the attended stimulus.
Treue, S. & Maunsell, J. H. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature 382, 539–541 (1996). This single-unit study demonstrates stronger and earlier effects of attention along the dorsal stream than previously reported.
Treue, S. & Maunsell, J. H. Effects of attention on the processing of motion in macaque middle temporal and medial superior temporal visual cortical areas. J. Neurosci. 19, 7591–7602 (1999).
Reynolds, J. H., Pasternak, T. & Desimone, R. Attention increases sensitivity of V4 neurons. Neuron 26, 703–714 (2000).
Williford, T. & Maunsell, J. H. Effects of spatial attention on contrast response functions in macaque area V4. J. Neurophysiol. 96, 40–54 (2006).
Luck, S. J., Chelazzi, L., Hillyard, S. A. & Desimone, R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J. Neurophysiol. 77, 24–42 (1997).
Motter, B. C. Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J. Neurophysiol. 70, 909–919 (1993).
Tootell, R. B. et al. The retinotopy of visual spatial attention. Neuron 21, 1409–1422 (1998).
Brefczynski, J. A. & DeYoe, E. A. A physiological correlate of the 'spotlight' of visual attention. Nature Neurosci. 2, 370–374 (1999).
Liu, T., Pestilli, F. & Carrasco, M. Transient attention enhances perceptual performance & FMRI response in human visual cortex. Neuron 45, 469–477 (2005).
Silver, M. A., Ress, D. & Heeger, D. J. Neural correlates of sustained spatial attention in human early visual cortex. J. Neurophysiol. 97, 229–237 (2007).
Datta, R. & DeYoe, E. A. I know where you are secretly attending! The topography of human visual attention revealed with fMRI. Vision Res. 49, 1037–1044 (2009).
Li, X., Lu, Z. L., Tjan, B. S., Dosher, B. A. & Chu, W. Blood oxygenation level-dependent contrast response functions identify mechanisms of covert attention in early visual areas. Proc. Natl Acad. Sci. USA 105, 6202–6207 (2008).
Buracas, G. T. & Boynton, G. M. The effect of spatial attention on contrast response functions in human visual cortex. J. Neurosci. 27, 93–97 (2007).
Pestilli, F., Carrasco, M., Heeger, D. J. & Gardner, J. L. Attentional enhancement viaselection and pooling of early sensory responses in human visual cortex. Neuron 72, 832–846 (2011).
Connor, C. E., Gallant, J. L., Preddie, D. C. & Van Essen, D. C. Responses in area V4 depend on the spatial relationship between stimulus and attention. J. Neurophysiol. 75, 1306–1308 (1996). This study provides the first evidence for shifts of a neuron's RF profile towards an attended stimulus.
Connor, C. E., Preddie, D. C., Gallant, J. L. & Van Essen, D. C. Spatial attention effects in macaque area V4. J. Neurosci. 17, 3201–3214 (1997).
Hamed, B. S., Duhamel, J. R., Bremmer, F. & Graf, W. Visual receptive field modulation in the lateral intraparietal area during attentive fixation and free gaze. Cereb. Cortex 12, 234–245 (2002).
Anton-Erxleben, K., Stephan, V. M. & Treue, S. Attention reshapes center-surround receptive field structure in macaque cortical area MT. Cereb. Cortex 19, 2466–2478 (2009).
Womelsdorf, T., Anton-Erxleben, K., Pieper, F. & Treue, S. Dynamic shifts of visual receptive fields in cortical area MT by spatial attention. Nature Neurosci. 9, 1156–1160 (2006). By measuring RF maps under different attentional conditions, this study demonstrates for the first time that RFs shift towards and shrink around an attended stimulus.
Womelsdorf, T., Anton-Erxleben, K. & Treue, S. Receptive field shift and shrinkage in macaque middle temporal area through attentional gain modulation. J. Neurosci. 28, 8934–8944 (2008).
Compte, A. & Wang, X. J. Tuning curve shift by attention modulation in cortical neurons: a computational study of its mechanisms. Cereb. Cortex 16, 761–778 (2006).
Miconi, T. & VanRullen, R. in Symp. on Computational Intelligence for Multimedia, Signal and Vision Processing 106–113 (IEEE Press, 2011).
Carrasco, M. & Yeshurun, Y. Covert attention effects on spatial resolution. Prog. Brain Res. 176, 65–86 (2009).
Carrasco, M. & Yeshurun, Y. The contribution of covert attention to the set-size and eccentricity effects in visual search. J. Exp. Psychol. Hum. Percept. Perform. 24, 673–692 (1998). This study shows for the first time that attention improves performance in feature and conjunction visual search and suggests that attention improves performance by enhancing spatial resolution.
Yeshurun, Y. & Rashal, E. Precueing attention to the target location diminishes crowding and reduces the critical distance. J. Vis. 10, 16 (2010).
Carrasco, M., Williams, P. E. & Yeshurun, Y. Covert attention increases spatial resolution with or without masks: support for signal enhancement. J. Vis. 2, 467–479 (2002).
Shalev, L. & Tsal, Y. Detecting gaps with and without attention: further evidence for attentional receptive fields. Eur. J. Cogn. Psychol. 14, 3–26 (2002). This study reports that attention improves performance in the detection and localization of a small gap and hypothesizes that attention increases resolution by decreasing the size of 'attentional RFs'.
Golla, H., Ignashchenkova, A., Haarmeier, T. & Thier, P. Improvement of visual acuity by spatial cueing: a comparative study in human and non-human primates. Vision Res. 44, 1589–1600 (2004). This study extends findings in humans to non-human primates and shows that, in both species, attention similarly improves performance in acuity tasks.
Yeshurun, Y. & Carrasco, M. The locus of attentional effects in texture segmentation. Nature Neurosci. 3, 622–627 (2000).
Carrasco, M., Loula, F. & Ho, Y. X. How attention enhances spatial resolution: evidence from selective adaptation to spatial frequency. Percept. Psychophys. 68, 1004–1012 (2006). Using selective adaptation to different spatial frequencies, this study attributes attention effects on texture segmentation performance to high spatial frequency-selective filters.
Abrams, J., Barbot, A. & Carrasco, M. Voluntary attention increases perceived spatial frequency. Atten. Percept. Psychophys. 72, 1510–1521 (2010). This study shows that attention alters the subjective appearance of a stimulus' spatial frequency. The authors suggest that this effect can be explained by a shift of sensitivity to higher spatial frequencies.
Gobell, J. & Carrasco, M. Attention alters the appearance of spatial frequency and gap size. Psychol. Sci. 16, 644–651 (2005).
Suzuki, S. & Cavanagh, P. Focused attention distorts visual space: an attentional repulsion effect. J. Exp. Psychol. Hum. Percept. Perform. 23, 443–463 (1997).
Anton-Erxleben, K., Henrich, C. & Treue, S. Attention changes perceived size of moving visual patterns. J. Vis. 7, 5 (2007). This study shows that attention alters the subjective appearance of the size of a stimulus, which is consistent with an attentional repulsion of the stimulus' borders. The authors suggest that this effect can be explained by an RF shift towards the attentional focus.
Fortenbaugh, F. C., Prinzmetal, W. & Robertson, L. C. Rapid changes in visual-spatial attention distort object shape. Psychon. Bull. Rev. 18, 287–294 (2011).
Treisman, A. M. & Gelade, G. A feature-integration theory of attention. Cogn. Psychol. 12, 97–136 (1980).
Treisman, A. M. Preattentive processing in vision. Comput. Vis. Graph. Image Process. 31, 156–177 (1985).
Nakayama, M. & Martini, P. Situating visual search. Vision Res. 51, 1526–1537 (2011).
Wolfe, J. M., Cave, K. R. & Franzel, S. L. Guided search: an alternative to the feature integration model for visual search. J. Exp. Psychol. Hum. Percept. Perform. 15, 419–433 (1989).
Nakayama, K. & Silverman, G. H. Serial and parallel processing of visual feature conjunctions. Nature 320, 264–265 (1986).
Duncan, J. & Humphreys, G. W. Visual search and stimulus similarity. Psychol. Rev. 96, 433–458 (1989).
McLeod, P., Driver, J. & Crisp, J. Visual search for a conjunction of movement and form is parallel. Nature 332, 154–155 (1988).
Enns, J. T. & Rensink, R. A. Influence of scene-based properties on visual search. Science 247, 721–723 (1990).
Carrasco, M. & Frieder, K. S. Cortical magnification neutralizes the eccentricity effect in visual search. Vision Res. 37, 63–82 (1997).
Carrasco, M., McLean, T. L., Katz, S. M. & Frieder, K. S. Feature asymmetries in visual search: effects of display duration, target eccentricity, orientation and spatial frequency. Vision Res. 38, 347–374 (1998).
Dosher, B., Han, S. & Lu, Z.-L. Time course of asymmetric visual search. J. Exp. Psychol. Hum. Percept. Perform. 30, 3–27 (2004).
Carrasco, M., Evert, D. L., Chang, I. & Katz, S. M. The eccentricity effect: target eccentricity affects performance on conjunction searches. Percept. Psychophys. 57, 1241–1261 (1995).
McElree, B. & Carrasco, M. The temporal dynamics of visual search: evidence for parallel processing in feature and conjunction searches. J. Exp. Psychol. Hum. Percept. Perform. 25, 1517–1539 (1999).
Carrasco, M., Ponte, D., Rechea, C. & Sampedro, M.J. “Transient structures”: the effects of practice and distractor grouping ion within-dimension conjunction searches. Percept. Psychophys. 60, 1243–1258 (1998).
Eckstein, M. Visual search: a retrospective. J. Vis. 11, 5 (2011).
Pelli, D. G. Crowding: a cortical constraint on object recognition. Curr. Opin. Neurobiol. 18, 445–451 (2008).
Mackeben, M. & Nakayama, K. Express attentional shifts. Vision Res. 33, 85–90 (1993).
Balz, G. W. & Hock, H. S. The effect of attentional spread on spatial resolution. Vision Res. 37, 1499–1510 (1997).
Lee, D. K., Koch, C. & Braun, J. Spatial vision thresholds in the near absence of attention. Vision Res. 37, 2409–2418 (1997).
Westheimer, G. Do ocular-dominance columns set spatial limits for hyperacuity processing? Vision Res. 22, 1349–1352 (1982).
Barlow, H. B. The Ferrier Lecture, 1980. Critical limiting factors in the design of the eye and visual cortex. Proc. R. Soc. Lond. B 212, 1–34 (1981).
Barlow, H. B. Reconstructing the visual image in space and time. Nature 279, 189–190 (1979).
Lee, D. K., Itti, L., Koch, C. & Braun, J. Attention activates winner-take-all competition among visual filters. Nature Neurosci. 2, 375–381 (1999).
Gurnsey, R., Pearson, P. & Day, D. Texture segmentation along the horizontal meridian: nonmonotonic changes in performance with eccentricity. J. Exp. Psychol. Hum. Percept. Perform. 22, 738–757 (1996).
Kehrer, L. Central performance drop on perceptual segregation tasks. Spat. Vis. 4, 45–62 (1989).
Kehrer, L. The central performance drop in texture segmentation: a simulation based on a spatial filter model. Biol. Cybern. 77, 297–305 (1997).
Potechin, C. & Gurnsey, R. Backward masking is not required to elicit the central performance drop. Spat. Vis. 16, 393–406 (2003).
Yeshurun, Y., Montagna, B. & Carrasco, M. On the flexibility of sustained attention and its effects on a texture segmentation task. Vision Res. 48, 80–95 (2008).
Talgar, C. P. & Carrasco, M. Vertical meridian asymmetry in spatial resolution: visual and attentional factors. Psychon. Bull. Rev. 9, 714–722 (2002).
Morikawa, K. Central performance drop in texture segmentation: the role of spatial and temporal factors. Vision Res. 40, 3517–3526 (2000).
Sperling, G. & Melchner, M. J. The attention operating characteristic: examples from visual search. Science 202, 315–318 (1978).
Kinchla, R. A. in Attention and Performance Vol. 3 (ed. Nikerson, R. S.) 213–238 (Erlbaum, 1980).
Pastukhov, A., Fischer, L. & Braun, J. Visual attention is a single, integrated resource. Vision Res. 49, 1166–1173 (2009).
Fortenbaugh, F. C. & Robertson, L. C. When here becomes there: attentional distribution modulates foveal bias in peripheral localization. Atten. Percept. Psychophys. 73, 809–828 (2011).
Pratt, J. & Turk-Browne, N. B. The attentional repulsion effect in perception and action. Exp. Brain Res. 152, 376–382 (2003).
McAdams, C. J. & Maunsell, J. H. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J. Neurosci. 19, 431–441 (1999). By measuring tuning curves with attention inside or outside the RF, this study shows that attention modulates neural activity via multiplicative scaling.
Reynolds, J. H., Chelazzi, L. & Desimone, R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J. Neurosci. 19, 1736–1753 (1999). This study shows that attention biases the response to two stimuli inside a neuron's RF in favour of the attended stimulus and proposes a model based on competitive interactions among neurons to account for these findings.
Lee, J. & Maunsell, J. H. Attentional modulation of MT neurons with single or multiple stimuli in their receptive fields. J. Neurosci. 30, 3058–3066 (2010).
Ghose, G. M. & Maunsell, J. H. Spatial summation can explain the attentional modulation of neuronal responses to multiple stimuli in area V4. J. Neurosci. 28, 5115–5126 (2008).
Niebergall, R., Khayat, P. S., Treue, S. & Martinez-Trujillo, J. C. Expansion of MT neurons excitatory receptive fields during covert attentive tracking. J. Neurosci. 31, 15499–15510 (2011).
Connor, C. E. Attention: beyond neural response increases. Nature Neurosci. 9, 1083–1084 (2006).
Salinas, E. & Abbott, L. F. Coordinate transformations in the visual system: how to generate gain fields and what to compute with them. Prog. Brain Res. 130, 175–190 (2001).
Fischer, J. & Whitney, D. Attention narrows position tuning of population responses in V1. Curr. Biol. 19, 1356–1361 (2009). By comparing the spread of the BOLD response for attended and unattended stimuli, this fMRI study shows that attention reduces the overlap of the representation of neighbouring stimuli in area V1, suggesting that attention enhances spatial resolution by sharpening spatial tuning.
Eurich, C. W. & Schwegler, H. Coarse coding: calculation of the resolution achieved by a population of large receptive field neurons. Biol. Cybern. 76, 357–363 (1997).
Treue, S. Climbing the cortical ladder from sensation to perception. Trends Cogn. Sci. 7, 469–471 (2003).
Hopf, J. M. et al. Direct neurophysiological evidence for spatial suppression surrounding the focus of attention in vision. Proc. Natl Acad. Sci. USA 103, 1053–1058 (2006).
Muller, N. G. & Kleinschmidt, A. The attentional 'spotlight's' penumbra: center-surround modulation in striate cortex. Neuroreport 15, 977–980 (2004).
Hopf, J. M., Boehler, C. N., Schoenfeld, M. A., Heinze, H. J. & Tsotsos, J. K. The spatial profile of the focus of attention in visual search: insights from MEG recordings. Vision Res. 50, 1312–1320 (2010).
Cutzu, F. & Tsotsos, J. K. The selective tuning model of attention: psychophysical evidence for a suppressive annulus around an attended item. Vision Res. 43, 205–219 (2003).
Allman, J., Miezin, F. & McGuinness, E. Direction- and velocity-specific responses from beyond the classical receptive field in the middle temporal visual area (MT). Perception 14, 105–126 (1985).
Born, R. T., Groh, J. M., Zhao, R. & Lukasewycz, S. J. Segregation of object and background motion in visual area MT: effects of microstimulation on eye movements. Neuron 26, 725–734 (2000).
Martinez-Trujillo, J. C. & Treue, S. Feature-based attention increases the selectivity of population responses in primate visual cortex. Curr. Biol. 14, 744–751 (2004).
Treue, S. & Martinez-Trujillo, J. C. Feature-based attention influences motion processing gain in macaque visual cortex. Nature 399, 575–579 (1999).
David, S. V., Hayden, B. Y., Mazer, J. A. & Gallant, J. L. Attention to stimulus features shifts spectral tuning of V4 neurons during natural vision. Neuron 59, 509–521 (2008).
Fritz, J., Shamma, S., Elhilali, M. & Klein, D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nature Neurosci. 6, 1216–1223 (2003).
Fritz, J. B., Elhilali, M. & Shamma, S. A. Differential dynamic plasticity of A1 receptive fields during multiple spectral tasks. J. Neurosci. 25, 7623–7635 (2005).
Lennie, P. Receptive fields. Curr. Biol. 13, 216–219 (2003).
Lennie, P. Single units and visual cortical organization. Perception 27, 889–935 (1998).
DeValois, R. L. & DeValois, K. K. Spatial Vision (Oxford Univ. Press, 1988).
Connolly, M. & Van Essen, D. The representation of the visual field in parvicellular and magnocellular layers of the lateral geniculate nucleus in the macaque monkey. J. Comp. Neurol. 226, 544–564 (1984).
Van Essen, D. C., Newsome, W. T. & Maunsell, J. H. The visual field representation in striate cortex of the macaque monkey: asymmetries, anisotropies, and individual variability. Vision Res. 24, 429–448 (1984).
Sereno, M. I. et al. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268, 889–893 (1995).
Tootell, R. B., Switkes, E., Silverman, M. S. & Hamilton, S. L. Functional anatomy of macaque striate cortex. II. Retinotopic organization. J. Neurosci. 8, 1531–1568 (1988).
Maunsell, J. H. & McAdams, C. J. in The New Cognitive Neurosciences (ed. Gazzaniga, M.) 315–324 (MIT Press, 1999).
Fix, J. et al. in Proc. of the 5th French Conference on Computational Neuroscience 147–152 (NeuroComp, 2010).
Olshausen, B. A., Anderson, C. H. & Van Essen, D. C. A neurobiological model of visual attention and invariant pattern recognition based on dynamic routing of information. J. Neurosci. 13, 4700–4719 (1993).
Desimone, R. & Duncan, J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 18, 193–222 (1995).
Reynolds, J. H. & Desimone, R. The role of neural mechanisms of attention in solving the binding problem. Neuron 24, 19–29, 111–125 (1999).
McDonald, J. J. & Green, J. J. Isolating event-related potential components associated with voluntary control of visuo-spatial attention. Brain Res. 1227, 96–109 (2008).
Hopfinger, J. B., Buonocore, M. H. & Mangun, G. R. The neural mechanisms of top-down attentional control. Nature Neurosci. 3, 284–291 (2000).
Lauritzen, T. Z., D'Esposito, M., Heeger, D. J. & Silver, M. A. Top-down flow of visual spatial attention signals from parietal to occipital cortex. J. Vis. 9, 18 (2009).
Rossi, A. F., Pessoa, L., Desimone, R. & Ungerleider, L. G. The prefrontal cortex and the executive control of attention. Exp. Brain Res. 192, 489–497 (2009).
Roberts, M., Delicato, L. S., Herrero, J., Gieselmann, M. A. & Thiele, A. Attention alters spatial integration in macaque V1 in an eccentricity-dependent manner. Nature Neurosci. 10, 1483–1491 (2007).
Ito, M. & Gilbert, C. D. Attention modulates contextual influences in the primary visual cortex of alert monkeys. Neuron 22, 593–604 (1999).
Sundberg, K. A., Mitchell, J. F. & Reynolds, J. H. Spatial attention modulates center-surround interactions in macaque visual area v4. Neuron 61, 952–963 (2009).
Acknowledgements
We thank S. Treue, Y. Yeshurun, B. Lawrence, the current members of the Carrasco laboratory and the two anonymous reviewers for helpful comments on the manuscript. This publication is supported by the US National Institutes of Health (NIH) grant NIH R01-EY019693 and NIH-R01-EY016200 (to M.C.), a Feodor-Lynen Research Fellowship, Alexander-von-Humboldt Foundation and NIH NRSA 1F32EY021420 (to K.A.-E.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Spatial resolution
-
The ability to discriminate two nearby points in space.
- Fovea
-
The central part of the retina with highest receptor density, finest receptor-to-retinal ganglion cell mapping and therefore best spatial resolution. It contains the highest density of cones and the highest cone-to-rod ratio.
- Extrastriate areas
-
All visual cortical areas that are higher in the processing hierarchy than primary visual cortex (V1; also known as striate cortex), including areas along the temporal (for example, area V4 and the inferior temporal cortex) as well as the dorsal pathway (for example, the medial temporal area and medial superior temporal area).
- Eccentricity
-
The distance from the fovea. Visual field eccentricity corresponds to retinal eccentricity during fixation.
- Spatial frequency tuning
-
The variation of the neuronal response to variations in spatial frequency of a stimulus. Spatial frequency describes the scale over which local contrast varies in a visual scene.
- Saccades
-
Rapid eye movements that align gaze with a new location in visual space several times per second.
- Spatial attention
-
Selection of a particular region in space so that processing of information from that location is enhanced. Here, we focus on visual spatial attention; that is, the selection of visual information and its effects on the activity of visual neurons and visual performance. Spatial attention can be directed overtly — that is, by moving the eyes towards the location of interest — or covertly — that is, without eye movements (covert attention). Covert attention can be allocated voluntarily (endogenous attention) or captured involuntarily (exogenous attention).
- Attentional focus
-
The location in the visual field at which attention is allocated.
- Cortical magnification factor
-
(CMF). The area of cortical surface to which a stimulus subtending 1 degree of visual angle on the retina projects. Often, the reciprocal of the CMF is used to determine the number of degrees visual angle a stimulus should subtend to activate 1mm of cortex. This number increases linearly with eccentricity.
- Landolt stimulus
-
A typical stimulus used to measure acuity. Observers have to detect a small gap in a circle or square or discriminate the location of the gap (for example, the left or right side of the circle or square).
- Vernier tasks
-
Typical tasks that are used to measure hyperacuity. Observers have to report a small lateral offset between two lines.
- Selectively adapting
-
Prolonged exposure to a particular stimulus selectively decreases the responses of neurons involved in processing the stimulus and therefore decreases sensitivity to the subsequent presentation of the same or a similar stimulus.
- Visual hemifields
-
These are one half of the visual field.
- Retinotopic maps
-
Neighbouring locations on the retina also stimulate neighbouring locations in retinotopic cortical areas. Thus, even though distances may be distorted (cortical magnification), spatial relations between different locations are kept from the retina through higher levels of the visual processing hierarchy, including the visual areas V1, V2 and V4, and the medial temporal area.
- Labelled-line code
-
The idea that information about a stimulus (for example, its location) in the nervous system is transmitted by activity in specific connections — 'labelled lines'. For example, receptive fields are 'labelled with their location in the visual field, and thus activity of a neuron with a certain receptive field creates the sensation of a stimulus at that particular location.
- Feature-based attention
-
The selection of a particular feature within a dimension, such as vertical orientation, red colour or upwards motion direction. Processing of the selected feature is enhanced independent of spatial location.
Rights and permissions
About this article
Cite this article
Anton-Erxleben, K., Carrasco, M. Attentional enhancement of spatial resolution: linking behavioural and neurophysiological evidence. Nat Rev Neurosci 14, 188–200 (2013). https://doi.org/10.1038/nrn3443
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3443
This article is cited by
-
Dissociable roles of human frontal eye fields and early visual cortex in presaccadic attention
Nature Communications (2023)
-
Presaccadic attention sharpens visual acuity
Scientific Reports (2023)
-
Tracking momentary fluctuations in human attention with a cognitive brain-machine interface
Communications Biology (2022)
-
Priority coding in the visual system
Nature Reviews Neuroscience (2022)
-
A dynamic normalization model of temporal attention
Nature Human Behaviour (2021)