Key Points
-
Two decades of clinical experience with the immunomodulatory treatment of multiple sclerosis points to distinct immunological pathways that drive disease relapses and progression. Although the immunomodulatory drugs reduce the frequency of relapses, the trade-off of efficacy is a range of side effects, and the long-standing drugs approved for multiple sclerosis do not ultimately halt neurodegeneration.
-
Dissecting the distinct roles of the immune system in multiple sclerosis is complicated by one, the multicellular pathophysiology that involves infiltrating adaptive and innate immune cells, as well as central nervous system (CNS)-resident innate cells with inflammatory capacity; and two, the chronic nature of the disease that unfolds over a period of many decades.
-
Multiple sclerosis is associated with more than 100 different genetic variants that promote disease predisposition and with environmental influences that alter disease penetrance and stochastic occurrences, although the exact triggering events may vary from one patient to the next. Despite the progress in identifying the genetic determinants of the disease, their phenotypic consequences remain to be elucidated, and a substantial understanding of environmental contributors is lacking.
-
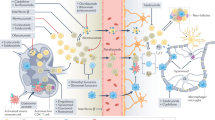
Dysregulation of immune effector–suppressor cell interactions occurs in multiple sclerosis, ultimately resulting in autoreactive adaptive immune cells that are capable of infiltrating and promoting damage within the CNS. However, these cells may not be the main drivers of more chronic, progressive neurodegeneration.
-
Chronic inflammation in multiple sclerosis may reflect a long-term stress response to homeostatic dysregulation in the CNS by tissue-resident innate cells that exceedingly burdens the system, leading to progressive and irreversible neurodegenerative decline.
-
The most imminent goal for future treatment is the concomitant improved targeting of relapses and progression, potentially through combinatorial therapies that modulate both arms of the disease. Improved disease prognosis and potential patient stratification for more directed healthcare provision are also much-anticipated prospects and may become tangible as we move into the immune informatics era and as large-scale, organized health resources become increasingly accessible.
Abstract
Two decades of clinical experience with immunomodulatory treatments for multiple sclerosis point to distinct immunological pathways that drive disease relapses and progression. In light of this, we discuss our current understanding of multiple sclerosis immunopathology, evaluate long-standing hypotheses regarding the role of the immune system in the disease and delineate key questions that are still unanswered. Recent and anticipated advances in the field of immunology, and the increasing recognition of inflammation as an important component of neurodegeneration, are shaping our conceptualization of disease pathophysiology, and we explore the potential implications for improved healthcare provision to patients in the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Compston, A. & Coles, A. Multiple sclerosis. Lancet 372, 1502–1517 (2008).
Kearney, H. et al. Cervical cord lesion load is associated with disability independently from atrophy in MS. Neurology 84, 367–373 (2015).
Frischer, J. M. et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 132, 1175–1189 (2009).
Haghikia, A., Hohlfeld, R., Gold, R. & Fugger, L. Therapies for multiple sclerosis: translational achievements and outstanding needs. Trends Mol. Med. 19, 309–319 (2013).
Feinstein, A., Freeman, J. & Lo, A. C. Treatment of progressive multiple sclerosis: what works, what does not, and what is needed. Lancet Neurol. 14, 194–207 (2015).
Friese, M. A., Schattling, B. & Fugger, L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat. Rev. Neurol. 10, 225–238 (2014).
Schattling, B. et al. TRPM4 cation channel mediates axonal and neuronal degeneration in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 18, 1805–1811 (2012).
Mayo, L. et al. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat. Med. 20, 1147–1156 (2014).
Popescu, B. F. & Lucchinetti, C. F. Pathology of demyelinating diseases. Annu. Rev. Pathol. 7, 185–217 (2012).
Beecham, A. H. et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat. Genet. 45, 1353–1360 (2013).
Brodin, P. et al. Variation in the human immune system is largely driven by non-heritable influences. Cell 160, 37–47 (2015). A comprehensive study of the contribution of non-genetic factors to the variation of more than 200 immunological parameters.
Belbasis, L., Bellou, V., Evangelou, E., Ioannidis, J. P. A. & Tzoulaki, I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 14, 263–273 (2015).
Harkiolaki, M. et al. T cell-mediated autoimmune disease due to low-affinity crossreactivity to common microbial peptides. Immunity 30, 348–357 (2009).
Muenz, C., Luenemann, J. D., Getts, M. T. & Miller, S. D. Antiviral immune responses: triggers of or triggered by autoimmunity? Nat. Rev. Immunol. 9, 246–258 (2009).
Olson, J. K., Croxford, J. L., Calenoff, M. A., Dal Canto, M. C. & Miller, S. D. A virus-induced molecular mimicry model of multiple sclerosis. J. Clin. Invest. 108, 311–318 (2001).
Ji, Q., Perchellet, A. & Goverman, J. M. Viral infection triggers central nervous system autoimmunity via activation of CD8+ T cells expressing dual TCRs. Nat. Immunol. 11, 628–634 (2010).
Ransohoff, R. M. & Engelhardt, B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 12, 623–635 (2012).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Heneka, M. T., Kummer, M. P. & Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 14, 463–477 (2014).
Gregory, A. P. et al. TNF receptor 1 genetic risk mirrors outcome of anti-TNF therapy in multiple sclerosis. Nature 488, 508–511 (2012). This study demonstrates how investigating the functional consequences of disease-associated genetic variation can help to predict therapeutic outcome.
Menche, J. et al. Uncovering disease-disease relationships through the incomplete interactome. Science 347, 1257601 (2015).
Tasan, M. et al. Selecting causal genes from genome-wide association studies via functionally coherent subnetworks. Nat. Methods 12, 154–159 (2015).
Farh, K. K. et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 518, 337–343 (2014).
Kundaje, A. et al. Integrative analysis of 111 reference human epigenomes. Nature 518, 317–330 (2015).
Raj, T. et al. Polarization of the effects of autoimmune and neurodegenerative risk alleles in leukocytes. Science 344, 519–523 (2014).
Ye, C. J. et al. Intersection of population variation and autoimmunity genetics in human T cell activation. Science 345, 1254665 (2014).
Roederer, M. et al. The genetic architecture of the human immune system: a bioresource for autoimmunity and disease pathogenesis. Cell 161, 387–403 (2015). An extensive immunophenotyping study that provides a bioresource for linking genetic control elements associated with normal immunological traits to common autoimmune and infectious diseases.
Friese, M. A. et al. Opposing effects of HLA class I molecules in tuning autoreactive CD8+ T cells in multiple sclerosis. Nat. Med. 14, 1227–1235 (2008).
Gregersen, J. W. et al. Functional epistasis on a common MHC haplotype associated with multiple sclerosis. Nature 443, 574–577 (2006).
Hartmann, F. J. et al. Multiple sclerosis-associated IL2RA polymorphism controls GM-CSF production in human TH cells. Nat. Commun. 5, 5056 (2014).
Dendrou, C. A. et al. Cell-specific protein phenotypes for the autoimmune locus IL2RA using a genotype-selectable human bioresource. Nat. Genet. 41, 1011–1015 (2009).
Gregory, S. G. et al. Interleukin 7 receptor α chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat. Genet. 39, 1083–1091 (2007).
Lundstrom, W. et al. Soluble IL7Rα potentiates IL-7 bioactivity and promotes autoimmunity. Proc. Natl Acad. Sci. USA 110, E1761–E1770 (2013).
Lambert, J.-C. et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat. Genet. 45, 1452–1458 (2013).
Nalls, M. A. et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nat. Genet. 46, 989–993 (2014).
Scally, S. W. et al. A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis. J. Exp. Med. 210, 2569–2582 (2013).
Miller, S. D. et al. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat. Med. 3, 1133–1136 (1997).
Quan, N., Whiteside, M. & Herkenham, M. Time course and localization patterns of interleukin-1β messenger RNA expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience 83, 281–293 (1998).
Vitkovic, L. et al. Cytokine signals propagate through the brain. Mol. Psychiatry 5, 604–615 (2000).
Dantzer, R., O'Connor, J. C., Freund, G. G., Johnson, R. W. & Kelley, K. W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–57 (2008).
Hedstrom, A. K., Akerstedt, T., Hillert, J., Olsson, T. & Alfredsson, L. Shift work at young age is associated with increased risk for multiple sclerosis. Ann. Neurol. 70, 733–741 (2011).
Operskalski, E. A., Visscher, B. R., Malmgren, R. M. & Detels, R. A case-control study of multiple sclerosis. Neurology 39, 825–829 (1989).
Lossius, A. et al. High-throughput sequencing of TCR repertoires in multiple sclerosis reveals intrathecal enrichment of EBV-reactive CD8+ T cells. Eur. J. Immunol. 44, 3439–3452 (2014).
Snyder, C. M. et al. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity 29, 650–659 (2008).
Duszczyszyn, D. A. et al. Thymic involution and proliferative T-cell responses in multiple sclerosis. J. Neuroimmunol. 221, 73–80 (2010).
Sargsyan, S. A. et al. Absence of Epstein-Barr virus in the brain and CSF of patients with multiple sclerosis. Neurology 74, 1127–1135 (2010).
Virgin, H. W. The virome in mammalian physiology and disease. Cell 157, 142–150 (2014).
Yatsunenko, T. et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012).
Berer, K. et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541 (2011).
Bielekova, B. et al. Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. J. Immunol. 172, 3893–3904 (2004).
Hellings, N. et al. T-cell reactivity to multiple myelin antigens in multiple sclerosis patients and healthy controls. J. Neurosci. Res. 63, 290–302 (2001).
McMahon, E. J., Bailey, S. L., Castenada, C. V., Waldner, H. & Miller, S. D. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat. Med. 11, 335–339 (2005).
Ji, Q., Castelli, L. & Goverman, J. M. MHC class I restricted myelin epitopes are cross-presented by Tip-DCs that promote determinant spreading to CD8+ T cells. Nat. Immunol. 14, 254–261 (2013).
Siewert, K. et al. Unbiased identification of target antigens of CD8+ T cells with combinatorial libraries coding for short peptides. Nat. Med. 18, 824–828 (2012).
Lutterotti, A. et al. Antigen-specific tolerance by autologous myelin peptide-coupled cells: a phase 1 trial in multiple sclerosis. Sci. Transl. Med. 5, 188ra175 (2013).
Kozovska, M. E. et al. Interferon beta induces T-helper 2 immune deviation in MS. Neurology 53, 1692–1697 (1999).
Miller, A. et al. Treatment of multiple sclerosis with copolymer-1 (Copaxone): implicating mechanisms of Th1 to Th2/Th3 immune-deviation. J. Neuroimmunol. 92, 113–121 (1998).
Zoghi, S. et al. Cytokine secretion pattern in treatment of lymphocytes of multiple sclerosis patients with fumaric acid esters. Immunol. Invest. 40, 581–596 (2011).
Frisullo, G. et al. IL17 and IFNγ production by peripheral blood mononuclear cells from clinically isolated syndrome to secondary progressive multiple sclerosis. Cytokine 44, 22–25 (2008).
Tzartos, J. S. et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 172, 146–155 (2008).
Cao, Y. et al. Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci. Transl. Med. 7, 287ra274 (2015).
Kebir, H. et al. Preferential recruitment of interferon-γ-expressing TH17 cells in multiple sclerosis. Ann. Neurol. 66, 390–402 (2009).
Segal, B. M. et al. Repeated subcutaneous injections of IL12/23 P40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 7, 796–804 (2008).
Ghoreschi, K. et al. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature 467, 967–971 (2010).
Zielinski, C. E. et al. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 484, 514–518 (2012).
Codarri, L. et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 12, 560–567 (2011).
Noster, R. et al. IL-17 and GM-CSF expression are antagonistically regulated by human T helper cells. Sci. Transl. Med. 6, 241ra280 (2014).
Sasaki, K. et al. Relapsing–remitting central nervous system autoimmunity mediated by GFAP-specific CD8 T cells. J. Immunol. 192, 3029–3042 (2014).
Willing, A. et al. CD8+ MAIT cells infiltrate into the CNS and alterations in their blood frequencies correlate with IL-18 serum levels in multiple sclerosis. Eur. J. Immunol. 44, 3119–3128 (2014).
Abrahamsson, S. V. et al. Non-myeloablative autologous haematopoietic stem cell transplantation expands regulatory cells and depletes IL-17 producing mucosal-associated invariant T cells in multiple sclerosis. Brain 136, 2888–2903 (2013).
Howell, O. W. et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 134, 2755–2771 (2011).
Drayton, D. L., Liao, S., Mounzer, R. H. & Ruddle, N. H. Lymphoid organ development: from ontogeny to neogenesis. Nat. Immunol. 7, 344–353 (2006).
Choi, S. R. et al. Meningeal inflammation plays a role in the pathology of primary progressive multiple sclerosis. Brain 135, 2925–2937 (2012).
Brickshawana, A. et al. Investigation of the KIR4.1 potassium channel as a putative antigen in patients with multiple sclerosis: a comparative study. Lancet Neurol. 13, 795–806 (2014).
Leypoldt, F., Armangue, T. & Dalmau, J. Autoimmune encephalopathies. Ann. NY Acad. Sci. 1338, 94–114 (2015).
Palanichamy, A. et al. Immunoglobulin class-switched B cells form an active immune axis between CNS and periphery in multiple sclerosis. Sci. Transl. Med. 6, 248ra106 (2014).
Stern, J. N. H. et al. B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Sci. Transl. Med. 6, 248ra107 (2014). References 76 and 77 use B cell receptor sequencing to investigate the relationship between B cell subsets in the periphery and the CNS, supporting the peripheral targeting of B cells for multiple sclerosis treatment.
Hauser, S. L. et al. B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N. Engl. J. Med. 358, 676–688 (2008).
Kappos, L. et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 378, 1779–1787 (2011).
Barr, T. A. et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J. Exp. Med. 209, 1001–1010 (2012).
Venken, K. et al. Natural naive CD4+CD25+CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. J. Immunol. 180, 6411–6420 (2008).
Martinez-Forero, I. et al. IL-10 suppressor activity and ex vivo Tr1 cell function are impaired in multiple sclerosis. Eur. J. Immunol. 38, 576–586 (2008).
Fritzsching, B. et al. Intracerebral human regulatory T cells: Analysis of CD4+CD25+FOXP3+ T cells in brain lesions and cerebrospinal fluid of multiple sclerosis patients. PLoS ONE 6, e17988 (2011).
Yogev, N. et al. Dendritic cells ameliorate autoimmunity in the CNS by controlling the homeostasis of PD-1 receptor+ regulatory T cells. Immunity 37, 264–275 (2012).
Roychoudhuri, R. et al. BACH2 represses effector programs to stabilize Treg-mediated immune homeostasis. Nature 498, 506–510 (2013).
Vahedi, G. et al. Super-enhancers delineate disease-associated regulatory nodes in T cells. Nature 520, 558–562 (2015).
Bennett, C. L. et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27, 20–21 (2001).
Venken, K. et al. Compromised CD4+CD25high regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology 123, 79–89 (2008).
Feger, U. et al. Increased frequency of CD4+CD25+ regulatory T cells in the cerebrospinal fluid but not in the blood of multiple sclerosis patients. Clin. Exp. Immunol. 147, 412–418 (2007).
Fletcher, J. M. et al. CD39+Foxp3+ regulatory T cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J. Immunol. 183, 7602–7610 (2009).
Dominguez-Villar, M., Baecher-Allan, C. M. & Hafler, D. A. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat. Med. 17, 673–675 (2011).
Schneider, A. et al. In active relapsing-remitting multiple sclerosis, effector T cell resistance to adaptive Tregs involves IL-6-mediated signaling. Sci. Transl. Med. 5, 170ra115 (2013).
Bhela, S. et al. Nonapoptotic and extracellular activity of granzyme B mediates resistance to regulatory T cell (Treg) suppression by HLA-DR−CD25hiCD127lo Tregs in multiple sclerosis and in response to IL-6. J. Immunol. 194, 2180–2189 (2015).
Hu, D. et al. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat. Immunol. 5, 516–523 (2004).
Pannemans, K. et al. HLA-E restricted CD8+ T cell subsets are phenotypically altered in multiple sclerosis patients. Mult. Scler. 20, 790–801 (2014).
Baughman, E. J. et al. Neuroantigen-specific CD8+ regulatory T-cell function is deficient during acute exacerbation of multiple sclerosis. J. Autoimmun. 36, 115–124 (2011).
Tennakoon, D. K. et al. Therapeutic induction of regulatory, cytotoxic CD8+ T cells in multiple sclerosis. J. Immunol. 176, 7119–7129 (2006).
Mattoscio, M. et al. Hematopoietic mobilization: potential biomarker of response to natalizumab in multiple sclerosis. Neurology 84, 1473–1482 (2015).
Schubert, R. D. et al. IFN-β treatment requires B cells for efficacy in neuroautoimmunity. J. Immunol. 194, 2110–2116 (2015).
Shen, P. et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507, 366–370 (2014).
Wherry, E. J. T cell exhaustion. Nat. Immunol. 12, 492–499 (2011).
Giannetti, P. et al. Increased PK11195-PET binding in normal-appearing white matter in clinically isolated syndrome. Brain 138, 110–119 (2015).
Kolasinski, J. et al. A combined post-mortem magnetic resonance imaging and quantitative histological study of multiple sclerosis pathology. Brain 135, 2938–2951 (2012).
Chard, D. T. et al. Brain atrophy in clinically early relapsing-remitting multiple sclerosis. Brain 125, 327–337 (2002).
Yamasaki, R. et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 211, 1533–1549 (2014). A study that used transgenic technology, scanning electron microscopy and gene expression profiling to demonstrate a differential role of microglia and monocytes in EAE.
Chen, Z. et al. Microglial displacement of inhibitory synapses provides neuroprotection in the adult brain. Nat. Commun. 5, 4486 (2014).
Neher, J. J. et al. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J. Immunol. 186, 4973–4983 (2011).
Goldmann, T. et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 16, 1618–1626 (2013).
Kotas, M. E. & Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 160, 816–827 (2015). An intriguing review on the parallels between homeostatic and inflammatory control mechanisms in health and disease.
Friese, M. A. et al. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat. Med. 13, 1483–1489 (2007).
Baruch, K. et al. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science 346, 89–93 (2014).
Kira, J. Neuromyelitis optica and Asian phenotype of multiple sclerosis. Ann. NY Acad. Sci. 1142, 58–71 (2008).
Fischer, M. T. et al. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 135, 886–899 (2012).
Shechter, R., London, A. & Schwartz, M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat. Rev. Immunol. 13, 206–218 (2013).
Perdiguero, E. G. et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518, 547–551 (2015).
Yednock, T. A. et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4β1 integrin. Nature 356, 63–66 (1992).
Ben-Nun, A. et al. From classic to spontaneous and humanized models of multiple sclerosis: impact on understanding pathogenesis and drug development. J. Autoimmun. 54, 33–50 (2014).
Campbell, G. R. et al. Clonally expanded mitochondrial DNA deletions within the choroid plexus in multiple sclerosis. Acta Neuropathol. 124, 209–220 (2012).
Haider, L. et al. Oxidative damage in multiple sclerosis lesions. Brain 134, 1914–1924 (2011).
Weisfeld-Adams, J. D., Katz Sand, I. B., Honce, J. M. & Lublin, F. D. Differential diagnosis of Mendelian and mitochondrial disorders in patients with suspected multiple sclerosis. Brain 138, 517–539 (2015).
Craner, M. J. et al. Molecular changes in neurons in multiple sclerosis: altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2+ exchanger. Proc. Natl Acad. Sci. USA 101, 8168–8173 (2004).
Acknowledgements
This work was supported by the Wellcome Trust, the Medical Research Council, the Alan and Babette Sainsbury Charitable Fund, and the Rosetrees Trust (L.F.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Approved drugs for the treatment of multiple sclerosis (PDF 134 kb)
Supplementary information S2 (table)
Murine EAE models. (PDF 180 kb)
PowerPoint slides
Glossary
- Demyelination
-
Damage to the myelin sheath surrounding nerves in the brain and spinal cord, which affects the function of the nerves involved. Demyelination occurs in multiple sclerosis and in experimental autoimmune encephalomyelitis, which is an animal model of multiple sclerosis.
- Gliosis
-
The proliferation and activation of glial cells (microglia, oligodendrocytes and astrocytes) in response to damage in the central nervous system.
- Tumefactive multiple sclerosis
-
A subtype of multiple sclerosis characterized by atypical, large demyelinated lesions that appear tumour-like and oedematous and can exert pressure on the surrounding central nervous system tissue due to their size.
- Molecular mimicry
-
A mechanism by which a peptide from a foreign antigen that is presented to a T cell closely resembles part of a self-protein, thereby triggering an autoimmune reaction.
- Choroid plexus
-
The site of production of cerebrospinal fluid in the adult brain. It is formed by invagination of ependymal cells into the ventricles, which become highly vascularized.
- Primary neurodegeneration
-
The process of progressive dysfunction and loss of axons and neurons, triggered by mechanisms involving central nervous system-resident cells, as opposed to cells infiltrating from the periphery.
- Candidate genes
-
Genes assumed to be affected by disease-associated genetic polymorphisms, based on their functional relevance and/or their physical proximity to the polymorphisms in question. The determination of whether the assigned candidates are truly affected by the polymorphisms and how they influence disease susceptibility typically requires functional follow-up investigations at the molecular, cellular and systemic levels.
- Interactome networks
-
Maps of molecular interactions, often segregated by cell type, and used as a framework to simplify cellular organization and to help address systems biology questions at the cellular level. These networks may reflect sets of physical intermolecular interactions as well as other molecules that indirectly act together in specific pathways.
- Central tolerance
-
Self-tolerance that is created at the level of the central lymphoid organs. Developing T cells (in the thymus) and B cells (in the bone marrow) that strongly recognize self-antigen must undergo further rearrangement of antigen-receptor genes to become self-tolerant, or they face deletion.
- Immune-privileged site
-
An area in the body with a decreased immune response to foreign antigens, including tissue grafts. These sites include the brain, eye, testis and placenta.
- Dural sinuses
-
Venous channels located between layers of the brain dura mater. These sinuses receive blood from both internal and external brain veins, and cerebrospinal fluid from the subarachnoid space, and empty into the jugular vein.
- Epitope spreading
-
This term is used to describe how a self-directed immune response induced by a single peptide (or epitope) could spread to include other peptides (or epitopes) not only on the same autoantigen (intramolecular spreading) but also on other self-molecules clustered in close vicinity within the target cell (intermolecular spreading).
- Diapedesis
-
The migration of leukocytes across the endothelium, which occurs by leukocytes squeezing through the junctions between adjacent endothelial cells.
- Cross-presentation
-
The initiation of a CD8+ T cell response to an antigen that is not present within antigen-presenting cells (APCs). This exogenous antigen must be taken up by APCs and then re-routed to the MHC class I pathway of antigen presentation.
- Mucosa-associated invariant T cells
-
(MAIT cells). A type of CD8+ T cell that is enriched at mucosal sites and is characterized by the expression of a semi-invariant T cell receptor (a dimer of Vα7.2 in combination with Jα12, Jα20 or Jα33) and is restricted by the non-polymorphic, highly evolutionarily conserved MHC class Ib molecule, MR1.
- Tertiary lymphoid structures
-
Organized lymphocytic aggregates that form in sites of chronic inflammation. Typically, B cell- and T cell-rich zones are segregated, and dendritic cells (DCs), germinal centres with follicular DC networks and specialized endothelial cells are present.
- Super-enhancer
-
A cluster of regulatory elements within a genomic region, often particularly enriched in sites that bind transcriptional co-activators.
- Immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome
-
(IPEX). A disease caused by mutations in FOXP3 (which encodes forkhead box P3) and characterized by refractory enteritis and, in some patients, autoimmune endocrinopathies, autoimmune diabetes and thyroiditis.
- Exhaustion
-
Non-responsiveness of the immune system resulting from the deletion of specific thymocytes (central tolerance) and the deletion or functional inactivation of specific T cells in the periphery (peripheral tolerance) in the presence of large quantities of antigen.
Rights and permissions
About this article
Cite this article
Dendrou, C., Fugger, L. & Friese, M. Immunopathology of multiple sclerosis. Nat Rev Immunol 15, 545–558 (2015). https://doi.org/10.1038/nri3871
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3871
This article is cited by
-
Border-associated macrophages in the central nervous system
Journal of Neuroinflammation (2024)
-
MIF contribution to progressive brain diseases
Journal of Neuroinflammation (2024)
-
Causal relationship between multiple sclerosis and cortical structure: a Mendelian randomization study
Journal of Translational Medicine (2024)
-
Helicobacter pylori infection and risk of multiple sclerosis: an updated meta-analysis
Neurological Sciences (2024)
-
Relapsing–remitting multiple sclerosis patients exhibit differential natural killer functional subpopulations
Acta Neurologica Belgica (2024)