Key Points
-
Fragile X syndrome is a monogenic neurodevelopmental disorder associated with intellectual disability and autism spectrum disorders.
-
Animal models have provided insights into the neurobiological mechanisms and enabled the identification of novel drug targets.
-
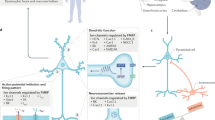
Promising targets include metabotropic glutamate receptor 5 (mGluR5), GABA receptors and proteins that are regulated or regulate fragile X mental retardation protein 1 (FMRP).
-
Many compounds have been extensively investigated in preclinical studies and are able to rescue altered levels of protein synthesis, synaptic plasticity and behaviour. Behavioural phenotypes in Fmr1-knockout mice are difficult to measure, and the rescue of these deficits has been inconsistent across these different drugs.
-
Subsequent clinical trials in humans were unable to demonstrate any improvement using behavioural measures as primary end points.
-
Objective measures of core phenotypes such as direct assessments of cognition and language rather than secondary behaviours need to be implemented in future trials.
-
Very young patients have not yet been included in clinical trials, and this may be the only group in which effects of a disease-modifying agent targeting cognition and development can be seen. Efforts may need to be redirected towards the implementation of longer trials in younger children accompanied by learning interventions measuring cognitive and developmental outcomes.
Abstract
Neurodevelopmental disorders such as fragile X syndrome (FXS) result in lifelong cognitive and behavioural deficits and represent a major public health burden. FXS is the most frequent monogenic form of intellectual disability and autism, and the underlying pathophysiology linked to its causal gene, FMR1, has been the focus of intense research. Key alterations in synaptic function thought to underlie this neurodevelopmental disorder have been characterized and rescued in animal models of FXS using genetic and pharmacological approaches. These robust preclinical findings have led to the implementation of the most comprehensive drug development programme undertaken thus far for a genetically defined neurodevelopmental disorder, including phase IIb trials of metabotropic glutamate receptor 5 (mGluR5) antagonists and a phase III trial of a GABAB receptor agonist. However, none of the trials has been able to unambiguously demonstrate efficacy, and they have also highlighted the extent of the knowledge gaps in drug development for FXS and other neurodevelopmental disorders. In this Review, we examine potential issues in the previous studies and future directions for preclinical and clinical trials. FXS is at the forefront of efforts to develop drugs for neurodevelopmental disorders, and lessons learned in the process will also be important for such disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Oberle, I. et al. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science 252, 1097–1102 (1991).
Pieretti, M. et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 66, 817–822 (1991). References 1 and 2 are seminal papers of the simultaneous discovery of FMR1 by several research groups.
Handt, M. et al. Point mutation frequency in the FMR1 gene as revealed by fragile X syndrome screening. Mol. Cell Probes 28, 279–283 (2014).
Dykens, E. M., Hodapp, R. M. & Leckman, J. F. Strengths and weaknesses in the intellectual functioning of males with fragile X syndrome. Am. J. Ment. Defic. 92, 234–236 (1987).
Fisch, G. S. et al. Longitudinal changes in cognitive-behavioral levels in three children with FRAXE. Am. J. Med. Genet. 84, 291–292 (1999).
Klaiman, C. et al. Longitudinal profiles of adaptive behavior in fragile X syndrome. Pediatrics 134, 315–324 (2014).
Baumgardner, T. L., Reiss, A. L., Freund, L. S. & Abrams, M. T. Specification of the neurobehavioral phenotype in males with fragile X syndrome. Pediatrics 95, 744–752 (1995).
Mazzocco, M. M., Pennington, B. F. & Hagerman, R. J. The neurocognitive phenotype of female carriers of fragile X: additional evidence for specificity. J. Dev. Behav. Pediatr. 14, 328–335 (1993).
Kaufmann, W. E., Abrams, M. T., Chen, W. & Reiss, A. L. Genotype, molecular phenotype, and cognitive phenotype: correlations in fragile X syndrome. Am. J. Med. Genet. 83, 286–295 (1999).
Sansone, S. M. et al. Improving IQ measurement in intellectual disabilities using true deviation from population norms. J. Neurodev. Disord. 6, 16 (2014).
de Vries, B. B. et al. Mental status of females with an FMR1 gene full mutation. Am. J. Hum. Genet. 58, 1025–1032 (1996).
Lewis, P. et al. Cognitive, language and social-cognitive skills of individuals with fragile X syndrome with and without autism. J. Intellect. Disabil. Res. 50, 532–545 (2006).
McDuffie, A. et al. Autism spectrum disorder in children and adolescents with fragile X syndrome: within-syndrome differences and age-related changes. Am. J. Intellect. Dev. Disabil. 115, 307–326 (2010).
Iossifov, I. et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 515, 216–221 (2014).
Darnell, J. C. et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261 (2011). This study identified a stringent set of 842 FMRP target transcripts, many of them associated with neuropsychiatric disorders. This highlights the fact that FMRP has broad regulatory roles at the synapse.
Hagerman, R. J., Murphy, M. A. & Wittenberger, M. D. A controlled trial of stimulant medication in children with the fragile X syndrome. Am. J. Med. Genet. 30, 377–392 (1988).
Ingrassia, A. & Turk, J. The use of clonidine for severe and intractable sleep problems in children with neurodevelopmental disorders — a case series. Eur. Child Adolesc. Psychiatry 14, 34–40 (2005).
Hagerman, R. J. et al. Advances in the treatment of fragile X syndrome. Pediatrics 123, 378–390 (2009).
Erickson, C. A., Stigler, K. A., Posey, D. J. & McDougle, C. J. Aripiprazole in autism spectrum disorders and fragile X syndrome. Neurotherapeutics 7, 258–263 (2010).
Bear, M. F., Huber, K. M. & Warren, S. T. The mGluR theory of fragile X mental retardation. Trends Neurosci. 27, 370–377 (2004). This is one of the seminal papers to propose a role for FMRP in the regulation of synaptic function and plasticity through regulation of long-term depression of synaptic strength involving stimulation of mGluR5. Excessive mGluR5 signalling has been targeted in several studies in rodents and humans.
Weiler, I. J. et al. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc. Natl Acad. Sci. USA 94, 5395–5400 (1997).
Ashley, C. T. Jr, Wilkinson, K. D., Reines, D. & Warren, S. T. FMR1 protein: conserved RNP family domains and selective RNA binding. Science 262, 563–566 (1993).
Li, Z. et al. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res. 29, 2276–2283 (2001).
Huber, K. M., Gallagher, S. M., Warren, S. T. & Bear, M. F. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl Acad. Sci. USA 99, 7746–7750 (2002).
Auerbach, B. D. & Bear, M. F. Loss of the fragile X mental retardation protein decouples metabotropic glutamate receptor dependent priming of long-term potentiation from protein synthesis. J. Neurophysiol. 104, 1047–1051 (2010).
Dölen, G. et al. Correction of fragile X syndrome in mice. Neuron 56, 955–962 (2007). This study demonstrates in the fragile X mouse model the rescue of a range of phenotypes relevant to the human disorder by reducing mGluR5 expression.
Gasparini, F. et al. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology 38, 1493–1503 (1999).
Porter, R. H. et al. Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J. Pharmacol. Exp. Ther. 315, 711–721 (2005).
Lindemann, L. et al. CTEP: a novel, potent, long-acting, and orally bioavailable metabotropic glutamate receptor 5 inhibitor. J. Pharmacol. Exp. Ther. 339, 474–486 (2011).
Gantois, I. et al. Chronic administration of AFQ056/Mavoglurant restores social behaviour in Fmr1 knockout mice. Behav. Brain Res. 239, 72–79 (2013).
Gross, C. et al. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J. Neurosci. 30, 10624–10638 (2010).
Michalon, A. et al. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron 74, 49–56 (2012). This study reported efficacy of a long-acting mGluR5 antagonist in adult Fmr1 -KO mice. The comprehensive phenotype correction after development of the phenotype sparked great interest for testing mGluR5 antagonists in adult and adolescent patients with FXS.
Osterweil, E. K., Krueger, D. D., Reinhold, K. & Bear, M. F. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J. Neurosci. 30, 15616–15627 (2010).
Pop, A. S. et al. Rescue of dendritic spine phenotype in Fmr1 KO mice with the mGluR5 antagonist AFQ056/Mavoglurant. Psychopharmacology 231, 1227–1235 (2014).
Yan, Q. J., Rammal, M., Tranfaglia, M. & Bauchwitz, R. P. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 49, 1053–1066 (2005).
Gassmann, M. & Bettler, B. Regulation of neuronal GABA(B) receptor functions by subunit composition. Nat. Rev. Neurosci. 13, 380–394 (2012).
Henderson, C. et al. Reversal of disease-related pathologies in the fragile X mouse model by selective activation of GABAB receptors with arbaclofen. Sci. Transl Med. 4, 152ra128 (2012).
Silverman, J. L. et al. GABAB receptor agonist R-baclofen reverses social deficits and reduces repetitive behavior in two mouse models of autism. Neuropsychopharmacology 40, 2228–2239 (2015).
Qin, M. et al. R-Baclofen reverses a social behavior deficit and elevated protein synthesis in a mouse model of fragile X syndrome. Int. J. Neuropsychopharmacol. 18, pyv034 (2015).
Mann, K., Kiefer, F., Spanagel, R. & Littleton, J. Acamprosate: recent findings and future research directions. Alcohol. Clin. Exp. Res. 32, 1105–1110 (2008).
Schaefer, T. L. et al. Acamprosate in a mouse model of fragile X syndrome: modulation of spontaneous cortical activity, ERK1/2 activation, locomotor behavior, and anxiety. J. Neurodev. Disord. 9, 6 (2017).
D'Hulst, C. et al. Expression of the GABAergic system in animal models for fragile X syndrome and fragile X associated tremor/ataxia syndrome (FXTAS). Brain Res. 1253, 176–183 (2009).
Braat, S. et al. The GABAA receptor is an FMRP target with therapeutic potential in fragile X syndrome. Cell Cycle 14, 2985–2995 (2015).
Carter, R. B. et al. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan-20-one), a selective, high-affinity, steroid modulator of the γ-aminobutyric acidA receptor. J. Pharmacol. Exp. Ther. 280, 1284–1295 (1997).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01725152 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01911455 (2016).
Mak, I. W., Evaniew, N. & Ghert, M. Lost in translation: animal models and clinical trials in cancer treatment. Am. J. Transl Res. 6, 114–118 (2014).
Lee, M. J. et al. Hedgehog pathway inhibitor saridegib (IPI-926) increases lifespan in a mouse medulloblastoma model. Proc. Natl Acad. Sci. USA 109, 7859–7864 (2012).
Bhattacharya, A. et al. Genetic removal of p70 S6 kinase 1 corrects molecular, synaptic, and behavioral phenotypes in fragile X syndrome mice. Neuron 76, 325–337 (2012).
Bilousova, T. V. et al. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J. Med. Genet. 46, 94–102 (2009).
Boda, B., Mendez, P., Boury-Jamot, B., Magara, F. & Muller, D. Reversal of activity-mediated spine dynamics and learning impairment in a mouse model of Fragile X syndrome. Eur. J. Neurosci. 39, 1130–1137 (2014).
Busquets-Garcia, A. et al. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat. Med. 19, 603–607 (2013).
Dolan, B. M. et al. Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by the small-molecule PAK inhibitor FRAX486. Proc. Natl Acad. Sci. USA 110, 5671–5676 (2013).
Gross, C. et al. Increased expression of the PI3K enhancer PIKE mediates deficits in synaptic plasticity and behavior in fragile X syndrome. Cell Rep. 11, 727–736 (2015).
Hayashi, M. L. et al. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc. Natl Acad. Sci. USA 104, 11489–11494 (2007).
Hebert, B. et al. Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by a BKCa channel opener molecule. Orphanet J. Rare Dis. 9, 124 (2014).
Thomas, A. M. et al. Genetic reduction of group 1 metabotropic glutamate receptors alters select behaviors in a mouse model for fragile X syndrome. Behav. Brain Res. 223, 310–321 (2011).
Tian, M. et al. 7, 8-Dihydroxyflavone induces synapse expression of AMPA GluA1 and ameliorates cognitive and spine abnormalities in a mouse model of fragile X syndrome. Neuropharmacology 89, 43–53 (2015).
Udagawa, T. et al. Genetic and acute CPEB1 depletion ameliorate fragile X pathophysiology. Nat. Med. 19, 1473–1477 (2013).
Laura, J., Stoppel, E. K. O. & Bear, M. F. in Fragile X Syndrome From Genetics to Targeted Treatment (eds Willemsen, R. & Kooy, F. R.) 173–204 (Academic Press, 2017).
Gross, C., Hoffmann, A., Bassell, G. J. & Berry-Kravis, E. M. Therapeutic strategies in fragile X syndrome: from bench to bedside and back. Neurotherapeutics 12, 584–608 (2015).
Kazdoba, T. M., Leach, P. T., Silverman, J. L. & Crawley, J. N. Modeling fragile X syndrome in the Fmr1 knockout mouse. Intractable Rare Dis. Res. 3, 118–133 (2014).
Leach, P. T., Hayes, J., Pride, M., Silverman, J. L. & Crawley, J. N. Normal performance of Fmr1 mice on a touchscreen delayed nonmatching to position working memory task. eNeuro http://dx.doi.org/10.1523/eneuro.0143-15.2016 (2016).
de Esch, C. E. et al. Fragile X mice have robust mGluR5-dependent alterations of social behaviour in the automated tube test. Neurobiol. Dis. 75, 31–39 (2015).
Westmark, C. J. et al. Reversal of fragile X phenotypes by manipulation of AβPP/Aβ levels in Fmr1KO mice. PLoS ONE 6, e26549 (2011).
Lindemann, L. et al. Pharmacology of basimglurant (RO4917523, RG7090), a unique metabotropic glutamate receptor 5 negative allosteric modulator in clinical development for depression. J. Pharmacol. Exp. Ther. 353, 213–233 (2015).
Vranesic, I. et al. AFQ056/mavoglurant, a novel clinically effective mGluR5 antagonist: identification, SAR and pharmacological characterization. Bioorg. Med. Chem. 22, 5790–5803 (2014).
Berry-Kravis, E. M. et al. Effects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: a randomized, controlled, phase 2 trial. Sci. Transl Med. 4, 152ra127 (2012).
Berry-Kravis, E. et al. Mavoglurant in fragile X syndrome: results of two randomized, double-blind, placebo-controlled trials. Sci. Transl Med. 8, 321ra325 (2016). This article presents the results of two of the largest randomized, placebo-controlled studies undertaken in adolescents and adults with FXS. These studies did not show efficacy of mavoglurant, but they have stimulated further study of mavoglurant in younger children with FXS.
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01483469 (2016).
Jacquemont, S. et al. Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Sci. Transl Med. 3, 64ra61 (2011).
Berry-Kravis, E. et al. Arbaclofen in fragile X syndrome: results of phase 3 trials. J. Neurodev. Disord. 9, 3 (2017).
Youssef, E. A. et al. Effect of the mGluR5-NAM basimglurant on behavior in adolescents and adults with fragile X syndrome in a randomized, double-blind, placebo-controlled trial: fragXis phase 2 results. Neuropsychopharmacology http://dx.doi.org/10.1038/npp.2017.177 (2017). This reference reports the large randomized, double-blind, placebo-controlled phase II trial with the mGluR5 antagonist basimglurant in adult and adolescent patients with FXS.
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01750957 (2016).
Caserta, M. S., Lund, D. A. & Wright, S. D. Exploring the caregiver burden inventory (CBI): further evidence for a multidimensional view of burden. Int. J. Aging Hum. Dev. 43, 21–34 (1996).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01015430 (2016).
Michalon, A. et al. Chronic metabotropic glutamate receptor 5 inhibition corrects local alterations of brain activity and improves cognitive performance in fragile X mice. Biol. Psychiatry 75, 189–197 (2014).
Silva-Santos, S. et al. Ube3a reinstatement identifies distinct developmental windows in a murine Angelman syndrome model. J. Clin. Invest. 125, 2069–2076 (2015).
Barnes, S. A. et al. Convergence of hippocampal pathophysiology in syngap+/− and Fmr1-/y mice. J. Neurosci. 35, 15073–15081 (2015).
Ehninger, D. et al. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat. Med. 14, 843–848 (2008).
Zu, T. et al. Recovery from polyglutamine-induced neurodegeneration in conditional SCA1 transgenic mice. J. Neurosci. 24, 8853–8861 (2004).
Radwan, B., Dvorak, D. & Fenton, A. A. Impaired cognitive discrimination and discoordination of coupled theta-gamma oscillations in Fmr1 knockout mice. Neurobiol. Dis. 88, 125–138 (2016).
Ethridge, L. E. et al. Reduced habituation of auditory evoked potentials indicate cortical hyper-excitability in fragile X syndrome. Transl Psychiatry 6, e787 (2016).
Scharf, S. H., Jaeschke, G., Wettstein, J. G. & Lindemann, L. Metabotropic glutamate receptor 5 as drug target for fragile X syndrome. Curr. Opin. Pharmacol. 20, 124–134 (2015).
Scott, S. et al. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph. Lateral Scler. 9, 4–15 (2008).
Dove, D. et al. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics 130, 717–726 (2012).
McCracken, J. T. et al. Risperidone in children with autism and serious behavioral problems. N. Engl. J. Med. 347, 314–321 (2002).
Aman, M. G. et al. Cognitive effects of risperidone in children with autism and irritable behavior. J. Child Adolesc. Psychopharmacol. 18, 227–236 (2008).
Dawson, G. et al. Randomized, controlled trial of an intervention for toddlers with autism: the early start denver model. Pediatrics 125, e17–e23 (2010).
Estes, A. et al. Long-term outcomes of early intervention in 6-year-old children with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry 54, 580–587 (2015).
Gillberg, C. et al. Long-term stimulant treatment of children with attention-deficit hyperactivity disorder symptoms. A randomized, double-blind, placebo-controlled trial. Arch. Gen. Psychiatry 54, 857–864 (1997).
Hessl, D. et al. A solution to limitations of cognitive testing in children with intellectual disabilities: the case of fragile X syndrome. J. Neurodev. Disord. 1, 33–45 (2009).
Berry-Kravis, E. et al. Development of an expressive language sampling procedure in fragile X syndrome: a pilot study. J. Dev. Behav. Pediatr. 34, 245–251 (2013).
McDuffie, A. et al. A spoken-language intervention for school-aged boys with fragile X syndrome. Am. J. Intellect. Dev. Disabil. 121, 236–265 (2016).
Knox, A. et al. Feasibility, reliability, and clinical validity of the test of attentional performance for children (KiTAP) in fragile X syndrome (FXS). J. Neurodev. Disord. 4, 2 (2012).
Curie, A. et al. A novel analog reasoning paradigm: new insights in intellectually disabled patients. PLoS ONE 11, e0149717 (2016).
Hessl, D. et al. The NIH toolbox cognitive battery for intellectual disabilities: three preliminary studies and future directions. J. Neurodev. Disord. 8, 35 (2016).
Wang, L. W., Berry-Kravis, E. & Hagerman, R. J. Fragile X: leading the way for targeted treatments in autism. Neurotherapeutics 7, 264–274 (2010).
Giles, L. L. & Martini, D. R. Challenges and promises of pediatric psychopharmacology. Acad. Pediatr. 16, 508–518 (2016).
Greiss Hess, L. et al. A randomized, double-blind, placebo-controlled trial of low-dose sertraline in young children with fragile X syndrome. J. Dev. Behav. Pediatr. 37, 619–628 (2016).
Brigman, J. L., Bussey, T. J., Saksida, L. M. & Rothblat, L. A. Discrimination of multidimensional visual stimuli by mice: intra- and extradimensional shifts. Behav. Neurosci. 119, 839–842 (2005).
Brigman, J. L., Graybeal, C. & Holmes, A. Predictably irrational: assaying cognitive inflexibility in mouse models of schizophrenia. Front. Neurosci. http://dx.doi.org/10.3389/neuro.01.013.2010 (2010).
Bussey, T. J. et al. New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology 62, 1191–1203 (2012).
Bussey, T. J. et al. The touchscreen cognitive testing method for rodents: how to get the best out of your rat. Learn. Mem. 15, 516–523 (2008).
van der Vaart, T., Overwater, I. E., Oostenbrink, R., Moll, H. A. & Elgersma, Y. Treatment of cognitive deficits in genetic disorders: a systematic review of clinical trials of diet and drug treatments. JAMA Neurol. 72, 1052–1060 (2015).
Cook, D. et al. Lessons learned from the fate of AstraZeneca's drug pipeline: a five-dimensional framework. Nat. Rev. Drug Discov. 13, 419–431 (2014).
Pammolli, F., Magazzini, L. & Riccaboni, M. The productivity crisis in pharmaceutical R&D. Nat. Rev. Drug Discov. 10, 428–438 (2011).
Curie, A. et al. Placebo responses in genetically determined intellectual disability: a meta-analysis. PLoS ONE 10, e0133316 (2015).
Jensen, K. B. et al. Certainty of genuine treatment increases drug responses among intellectually disabled patients. Neurology 88, 1912–1918 (2017).
Punja, S. et al. N-Of-1 trials are a tapestry of heterogeneity. J. Clin. Epidemiol. 76, 47–56 (2016).
Gabler, N. B., Duan, N., Vohra, S. & Kravitz, R. L. N-Of-1 trials in the medical literature: a systematic review. Med. Care 49, 761–768 (2011).
US Food and Drug Administration. Specific requirements on content and format of labelling for human prescription drugs: revision of 'pediatric' use' subsection in the labelling: final rule. FDA https://www.fda.gov/ohrms/dockets/ac/01/briefing/3778b1_Tab6_7-21CFR%20Part%20201.pdf (1994).]
European Medicines Agency. ICH topic E 11 clinical investigation of medicinal products in the paediatric population. European Medicines Agency http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002926.pdf (2001).
European Medicines Agency. Extrapolation of efficacy and safety in paediatric medicine development. European Medicines Agency http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_001367.jsp&mid=WC0b01ac0580029572 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02920892 (2017).
European Medicines Agency. Guideline on the clinical development of medicinal products for the treatment of autism spectrum disorder (ASD). European Medicines Agency http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/03/WC500202650.pdf (2013).
US Food and Drug Administration. Pharmacology/toxicology. FDA http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm065014.htm (2017).
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Safety guidelines. ICH http://www.ich.org/products/guidelines/safety/article/safety-guidelines.html (2017).
US Food and Drug Administration. Guidance for industry: nonclinical safety evaluation of pediatric drug products. FDA http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm079247.pdf (2006).
National Academies of Sciences, Engineering and Medicine. Neuroscience Trials of the Future: Proceedings of a Workshop (The National Academies Press, 2016).
Innovative Medicines Initiative. IMI2 10 th call for proprosals. Innovative Medicines Initiative http://www.imi.europa.eu/sites/default/files/uploads/documents/IMI2Call10/IMI2_Call10_TopicsText.pdf (2016).
Osterweil, E. K. et al. Lovastatin corrects excess protein synthesis and prevents epileptogenesis in a mouse model. Neuron 77, 234–250 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02642653 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02680379 (2016).
Gantois, I. et al. Metformin ameliorates core deficits in a mouse model of fragile X syndrome. Nat. Med. 23, 674–677 (2017).
Sidhu, H., Dansie, L. E., Hickmott, P. W., Ethell, D. W. & Ethell, I. M. Genetic removal of matrix metalloproteinase 9 rescues the symptoms of fragile X syndrome in a mouse model. J. Neurosci. 34, 9867–9879 (2014).
Chiu, C. T. & Chuang, D. M. Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacol. Ther. 128, 281–304 (2010).
Liu, Z. H. et al. Lithium reverses increased rates of cerebral protein synthesis in a mouse model of fragile x syndrome. Neurobiol. Dis. 45, 1145–1152 (2012).
Yuskaitis, C. J. et al. Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndrome. Biochem. Pharmacol. 79, 632–646 (2010).
Berry-Kravis, E. et al. Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome. J. Dev. Behav. Pediatr. 29, 293–302 (2008).
Braithwaite, S. P. et al. Synaptic plasticity: one STEP at a time. Trends Neurosci. 29, 452–458 (2006).
Goebel-Goody, S. M. et al. Taking STEPs forward to understand fragile x syndrome. Results Probl. Cell Differ. 54, 223–241 (2012).
Goebel-Goody, S. M. et al. Genetic manipulation of STEP reverses behavioral abnormalities in a fragile X syndrome mouse model. Genes Brain Behav. 11, 586–600 (2012).
Klann, E. & Dever, T. E. Biochemical mechanisms for translation regulation in synaptic plasticity. Nat. Rev. Neurosci. 5, 931–942 (2004).
Narayanan, U. et al. S6K1 phosphorylates and regulates fragile X mental retardation protein (FMRP) with the neuronal protein synthesis-dependent mammalian target of rapamycin (mTOR) signaling cascade. J. Biol. Chem. 283, 18478–18482 (2008).
Bhattacharya, A. et al. Targeting translation control with p70 S6 kinase 1 inhibitors to reverse phenotypes in fragile X syndrome mice. Neuropsychopharmacology 41, 1991–2000 (2016).
Kano, M. et al. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 89, 309–380 (2009).
Gomis-Gonzalez, M., Matute, C., Maldonado, R., Mato, S. & Ozaita, A. Possible therapeutic doses of cannabinoid type 1 receptor antagonist reverses key alterations in fragile X syndrome mouse model. Genes 7, E56 (2016).
Xie, S. et al. The endocannabinoid system and rimonabant: a new drug with a novel mechanism of action involving cannabinoid CB1 receptor antagonism — or inverse agonism — as potential obesity treatment and other therapeutic use. J. Clin. Pharm. Ther. 32, 209–231 (2007).
Christensen, R. et al. Efficacy and safety of the weight-loss drug rimonabrant: a meta analysis of randomised trials. Lancet 370, 1706–1713 (2007).
[No authors listed.] Rimonabant: suicide and depression. Depression and suicidal tendencies are about twice as frequent with rimonabant as with placebo. Prescrire Int. 16, 250 (2007).
Chen, L. Y. et al. Physiological activation of synsptic Rac>PAK (p-21activated kinase) signalling is defective in a mouse model of fragile X syndrome. J. Neurosci. 30, 10977–10984 (2010).
Henley, J. M. & Wilkinson, K. A. Synaptic AMPA receptor composition in development, plasticity and disease. Nat. Rev. Neurosci. 17, 337–350 (2016).
Lee, K. et al. AMPA receptors as therapeutic targets for neurological disorders. Adv. Protein Chem. Struct. Biol. 103, 203–261 (2016).
Arai, A. C. & Kessler, M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr. Drug Targets. 8, 583–602 (2007).
Li, J. et al. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol. Cell. Neurosci. 19, 138–151 (2002).
Berry-Kravis, E. et al. Effect of CX516, an AMPA-modulating compound, on cognition and behavior in fragile X syndrome: a controlled trial. J. Child Adolesc. Psychopharmacol. 16, 525–540 (2006).
Davenport, M. H., Schaefer, T. L., Friedmann, K. J., Fitzpatrick, S. E. & Erickson, C. A. Pharmacotherapy for fragile X syndrome: progress to date. Drugs 76, 431–445 (2016).
Ligsay, A. & Hagerman, R. J. Review of targeted treatments in fragile X syndrome. Intractable Rare Dis. Res. 5, 158–167 (2016).
Berry-Kravis, E. et al. A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. J. Med. Genet. 46, 266–271 (2009).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01348087 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01433354 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01894958 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02126995 (2016).
Paribello, C. et al. Open-label add-on treatment trial of minocycline in fragile X syndrome. BMC Neurol. 10, 91 (2010).
Leigh, M. J. et al. A randomized double-blind, placebo-controlled trial of minocycline in children and adolescents with fragile x syndrome. J. Dev. Behav. Pediatr. 34, 147–155 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01013480 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01555333 (2013).
Erickson, C. A. et al. Impact of acamprosate on behavior and brain-derived neurotrophic factor: an open-label study in youth with fragile X syndrome. Psychopharmacology 228, 75–84 (2013).
Kesler,S. R., Lightbody, A. A. & Reiss, A. L. et al. Cholinergic dysfunction in fragile X syndrome and potenial intervention. Am. J. Med. Genet. A 149A, 403–407 (2012).
Ligsay, A. et al. A randomized double-blind, placebo-controlled trial of ganaxolone in children and adolescents with fragile X syndrome. J. Neurodev. Disord. 9, 26 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01120626 (2016).
Sahu, J. K. et al. Effectiveness and safety of donepezil in boys with fragile x syndrome: a double-blind, randomized, controlled pilot study. J. Child Neurol. 28, 570–575 (2013).
Acknowledgements
The authors acknowledge the patients, caregivers and global fragile X syndrome community of scientists and investigators who contribute to the body of knowledge that continues to inform the treatment and care of this special patient population. The authors thank Spectrum and artist N. Rapp for Fig. 2. Selected research leading to these results has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement number 115300, resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007–2013) and EFPIA companies' in kind contribution. V.D.P. and A.C. thank the Clinical Center of Investigation (CIC), 1407 INSERM, Hospices Civils de Lyon, 69002, Lyon, France. Some work presented here has been facilitated by the Fragile X Clinical and Research Consortium (FXCRC) in the USA. E.B.K. is supported by U01NS096767. A.E.J. is the recipient of grants from Odense University Hospital Free Research Fund (grant number 15-A857) and of the Region of Zealand and Region of Southern of Denmark joint research fund (journal no. 14-001308) as well as a PhD scholarship from the Region of Southern of Denmark and the Faculty of Health Sciences, University of Southern of Denmark. M.F.B. acknowledges past and ongoing support and encouragement from FRAXA and NIH grant number R01-MH106469. J.N.C. is the recipient of NIH grant numbers R01NS085709 and U54HD079125-04 and Autism Speaks PACT targeted grant number 8603. E.L. is supported by EU-AIMS (European Autism Interventions), which receives support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115300, the resources of which are composed of financial contributions from the European Union's Seventh Framework Programme (grant FP7/2007-2013), from the European Federation of Pharmaceutical Industries and Associations. R.H. is funded by The MIND Institute IDDRC U54 HD079125, HRSA grants R40MC22641 and R40MC27701 and DOD PR101054. S.J. is the recipient of a Bursary Professor fellowship of the Swiss National Science Foundation (SNSF), the Jeanne et Jean Louis Levesque Research Chair and the Canadian Research Chair.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
E.B.K. has received funding from Novartis, Roche, Alcobra, Neuren, Cydan, Fulcrum, GW, Marinus, Edison and Neurotrope Pharmaceuticals to consult on trial design and development strategies and/or conduct clinical trials in fragile X syndrome (FXS), Vtesse to run clinical trials in Niemann–Pick type C, and from Asuragen Inc. to develop testing standards for FMR1 testing. L.L. is a full-time employee of F. Hoffmann-La Roche AG, he owns stock and stock options in F. Hoffmann-La Roche AG, and he is a co-inventor on patents originating in his work at F. Hoffmann-La Roche AG. A.E.J. participated in clinical trials led by Novartis Pharmaceuticals and has no further competing interests to declare. G.A. was a former full-time employee of Novartis Pharma and is currently an employee of Shire Pharmaceuticals. M.F.B. declares no competing interests. R.L.C. was a former full-time employee of Seaside Therapeutics and declares no further competing interests. J.N.C. declares no competing interests. A.C. participated in clinical trials led by Novartis Pharma and has no further competing interests to declare. V.D.P. participated in clinical trials led by Novartis Pharma and has no further competing interests to declare. F.H. is a former employee of Novartis Pharmaceutical Corporation and is currently employed by Celgene. F.G. is an employee and a shareholder of Novartis. B.G.M. is an employee of Novartis Institutes for Biomedical Research. D.H. has provided paid consultation regarding clinical trials in FXS to Novartis, Roche and Seaside Therapeutics. E.L. declares no competing interests. S.H.S. is a full-time employee of F. Hoffmann-La Roche AG. P.W. declares no competing interests. F.V.R. is a full-time employee and shareholder of Novartis Pharma. R.H. has received funding from Novartis, Roche/Genentech, Alcobra, Marinus and Neuren for clinical trials in FXS and has consulted with Roche/Genentech, Zynerba and Novartis regarding fragile X treatment and clinical trial design. W.S. is an employee of F. Hoffman-La Roche. S.J. has served on the Novartis Fragile X Advisory Board and has consulted for Novartis and WG Pharma.
Glossary
- Mosaicism
-
The presence of cell populations with a full mutation or premutation expansions. Methylation mosaicism is defined as some cells carrying fully methylated alleles and others carrying unmethylated alleles. Approximately 40% of male patients with fragile X syndrome present with size-mosaicism.
- Rotarod
-
A performance test in which the rodent is placed on a rotating rod to examine motor skills and coordination.
- Aberrant Behaviour Checklist
-
(ABC). A caregiver-rated symptom checklist that assesses problem behaviours via a 58-item and 5-subscale questionnaire. Each item is attributed a score from 0 (“not at all a problem”) to 3 (“problem is severe in degree”), resulting in total score ranks from 0 to 174.
- Endophenotypes
-
Phenotypes that bear a closer relationship to the biological processes underlying the clinical manifestation.
- ABC-CFX Social Avoidance subscale
-
The ABC-CFX is a modified version of the ABC-C, with 55 items and 6 subscales (irritability, lethargy/withdrawal, stereotypic behaviour, hyperactivity, inappropriate speech and social avoidance). The total score ranks from 0 to165, and a negative change from baseline indicates improvement.
- Vineland Adaptive Behavior Scale
-
(VABS). A test that measures adaptive behaviour across lifespan and contains five domains (communication, daily living skills, socialization, motor skills and maladaptive behaviour) each with 2–3 subdomains, such as expressive language.
- Audiogenic seizures
-
Convulsions caused by prolonged exposure to high frequency sound in, for example, rodents.
- National Institutes of Health Toolbox
-
A battery of extensively validated computer-administered cognitive, emotional, motor and sensory tests with utility across the lifespan.
- Cancellation task
-
A test of attention span in which the participants cancel the target figure and leave all other figures uncancelled (in other words, it is a test of the number of correct detections).
- Wechsler Intelligence Scale for Children
-
(WISC). An intelligence test for children between 6 and 16 years of age.
- Stanford–Binet
-
A cognitive ability and intelligence test used for individuals aged 2 to 85+ years.
- Jadad score
-
A score that ranks the quality of clinical trials with respect to randomization, blinding and placebo control on a score from 0–5, with 5 being the maximum score.
- Open label
-
A type of clinical trial in which the treatment being administered is known to both the researchers and participants.
- Bayesian Design trials
-
A theory of statistical inference in clinical trials.
- Sequential studies
-
Studies that combine longitudinal and cross-sectional designs by following several different age cohorts over time.
- Test–retest validation
-
A measure of reliability obtained by administering the same test twice over a period of time to a group of individuals.
Rights and permissions
About this article
Cite this article
Berry-Kravis, E., Lindemann, L., Jønch, A. et al. Drug development for neurodevelopmental disorders: lessons learned from fragile X syndrome. Nat Rev Drug Discov 17, 280–299 (2018). https://doi.org/10.1038/nrd.2017.221
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2017.221
This article is cited by
-
Characterising the behaviours in most severe and least severe emotional outbursts in young people
Scientific Reports (2024)
-
Latent Class Analysis Identifies Distinctive Behavioral Subtypes in Children with Fragile X Syndrome
Journal of Autism and Developmental Disorders (2024)
-
Role of the endocannabinoid system in fragile X syndrome: potential mechanisms for benefit from cannabidiol treatment
Journal of Neurodevelopmental Disorders (2023)
-
Modeling SHANK3-associated autism spectrum disorder in Beagle dogs via CRISPR/Cas9 gene editing
Molecular Psychiatry (2023)
-
Expressive language sampling and outcome measures for treatment trials in fragile X and down syndromes: composite scores and psychometric properties
Scientific Reports (2023)