Abstract
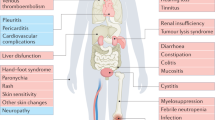
Cancer therapy makes patients sick. The therapies that are available to clinicians allow them to successfully control nausea, emesis and pain. However, this is not the case for a number of other symptoms that include fatigue, distractibility, poor memory, and diminished interest in previously pleasurable activities. These symptoms cluster during the course of cancer therapy and impair patient quality of life, limit therapy options and do not always resolve at the cessation of treatment. It is possible to describe the intensity and temporal features of symptoms and assess their relationship with the inflammatory response that is associated with cancer and cancer therapy. At the preclinical level, sophisticated animal models still need to be deployed to study the causal role of inflammation in specific components of cancer-related symptoms. Various approaches can be optimally combined in a translational symptom research pathway to provide a framework for assessing in a systematic manner the neurobehavioral toxicity of existing and newly developed cancer therapies. Ultimately, this knowledge will allow derivation of mechanism-based interventions to prevent or alleviate cancer-related symptoms.
Key Points
-
Overwhelming fatigue, distractibility, poor memory, and lack of interest in activities that used to be pleasurable are among the most distressing symptoms patients experience in response to cancer therapy
-
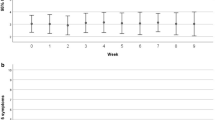
The trajectory of these symptoms can be studied over time together with their co-occurrence in the form of clusters and their impact on patients' daily functioning
-
There is already evidence that the development of cancer-related symptoms is associated with an increased expression of inflammatory mediators
-
Animal studies have confirmed that cancer therapy induces profound neurobehavioural changes that are associated with inflammation; attempts to model key symptoms in cancer patients are still scarce
-
Individual differences in symptom severity in response to cancer therapy provide a unique perspective on the genomics of symptoms that should be incorporated in animal models of cancer-related symptoms
-
Preclinical and clinical approaches can be combined in a translational research pathway that provides a framework for addressing the neurobehavioural toxicities of cancer therapies
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Cleeland, C. S. et al. Pain and its treatment in outpatients with metastatic cancer. N. Engl. J. Med. 330, 592–596 (1994).
Fisch, M. J. et al. Prospective observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung, or prostate cancer. J. Clin. Oncol. http://dx.doi.org/10.1200/JCO.2011.39.2381
Cleeland, C. S. Cancer-related symptoms. Semin. Radiat. Oncol. 10, 175–190 (2000).
Shi, Q. et al. Symptom burden in cancer survivors 1 year after diagnosis: a report from the American Cancer Society's Studies of Cancer Survivors. Cancer 117, 2779–2790 (2011).
Cleeland, C. S., O'Mara, A., Zagari, M. & Baas, C. Integrating pain metrics into oncology clinical trials. Clin. Cancer Res. 17, 6646–6650 (2011).
Chang, Y. J. et al. Assessment of clinical relevant fatigue level in cancer. Support. Care Cancer 15, 891–896 (2007).
Segota, E. & Bukowski, R. M. The promise of targeted therapy: cancer drugs become more specific. Cleve. Clin. J. Med. 71, 551–560 (2004).
Shepard, D. R. & Garcia, J. A. Toxicity associated with the long-term use of targeted therapies in patients with advanced renal cell carcinoma. Expert Rev. Anticancer Ther. 9, 795–805 (2009).
Miller, A. H., Ancoli-Israel, S., Bower, J. E., Capuron, L. & Irwin, M. R. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J. Clin. Oncol. 26, 971–982 (2008).
Cleeland C. S. & Sloan, J. A. ASCPRO Organizing Group. Assessing the symptoms of cancer using patient-reported outcomes (ASCPRO): searching for standards. J. Pain Symptom Manage. 39, 1077–1085 (2010).
Mendoza, T. R. et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 85, 1186–1196 (1999).
Cleeland, C. S. et al. Assessing symptom distress in cancer patients: the, M.D. Anderson Symptom Inventory. Cancer 89, 1634–1646 (2000).
Wang, X. S. et al. Longitudinal study of the relationship between chemoradiation therapy for non-small-cell lung cancer and patient symptoms. J. Clin. Oncol. 24, 4485–4491 (2006).
Cleeland, C. S. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J. Natl Cancer Inst. Monogr. 37, 16–21 (2007).
Dodd, M. J., Miaskowski, C. & Paul, S. M. Symptom clusters and their effect on the functional status of patients with cancer. Oncol. Nurs. Forum 28, 465–470 (2001).
Kirkova, J., Aktas, A., Walsh, D. & Davis, M. P. Cancer symptom clusters: clinical and research methodology. J. Palliat. Med. 14, 1149–1166 (2011).
Nelson, C. J., Nandy, N. & Roth, A. J. Chemotherapy and cognitive deficits: mechanisms, findings, and potential interventions. Palliat. Support. Care 5, 273–280 (2007).
Cleeland, C. S. et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer 97, 2919–2925 (2003).
Kim, H. J., Barsevick, A. M., Fang, C. Y. & Miaskowski, C. Common biological pathways underlying the psychoneurological symptom cluster in cancer patients. Cancer Nurs. http://dx.doi.org/10.1097/NCC.0b013e318233a811.
Wang, X. S. et al. Serum sTNF-R1 and IL-6 are associated with severity of symptom burden caused by concurrent chemoradiation therapy for patients with lung and gastrointestinal cancers. Brain Behav. Immun. 32 (Suppl. 2), S61–S62 (2009).
Shi, Q. & Cleeland, C. S. in Cancer Symptom Science: Measurement and Management (eds Cleeland, C. S., Fisch, M. J. & Dunn, A. J.) 192–205 (Cambridge University Press, Cambridge, 2011).
Pacharinsak, C. & Beitz, A. Animal models of cancer pain. Comp. Med. 58, 220–233 (2008).
Chang, V. T. & Portenoy, R. K. in Cancer Symptom Science: Measurement and Management (eds Cleeland, C. S., Fisch, M. J. & Dunn, A. J.) 18–30 (Cambridge University Press, Cambridge, 2011).
Zhang, H. & Dougherty, P. M. in Cancer Symptom Science: Measurement and Management (eds Cleeland, C. S., Fisch, M. J. & Dunn, A. J.) 41–50 (Cambridge University Press, Cambridge, 2011).
Dantzer, R. Cytokine-induced sickness behavior: where do we stand? Brain Behav. Immun. 15, 7–24 (2001).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Wortel, C. H. et al. Interleukin-6 mediates host defense responses induced by abdominal surgery. Surgery 114, 564–570 (1993).
Schmidt, A., Bengtsson, A., Tylman, M. & Blomqvist, L. Pro-inflammatory cytokines in elective flap surgery. J. Surg. Res. 137, 117–121 (2007).
Deiner, S. & Silverstein, J. H. Postoperative delirium and cognitive dysfunction. Br. J. Anaesth. 103 (Suppl. 1), i41–i46 (2009).
Cibelli, M. et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann. Neurol. 68, 360–368 (2010).
Brode, S. & Cooke, A. Immune-potentiating effects of the chemotherapeutic drug cyclophosphamide. Crit. Rev. Immunol. 28, 109–126 (2008).
Hei, T. K., Zhou, H., Chai, Y., Ponnaiya, B. & Ivanov, V. N. Radiation induced non-targeted response: mechanism and potential clinical implications. Curr. Mol. Pharmacol. 4, 96–105 (2011).
Tsavaris, N., Kosmas, C., Vadiaka, M., Kanelopoulos, P. & Boulamatsis, D. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br. J. Cancer 87, 21–27 (2002).
Bower, J. E. et al. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J. Clin. Oncol. 29, 3517–3522 (2011).
Hann, D. M. et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual. Life Res. 7, 301–310 (1998).
Bower, J. E. et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin. Cancer Res. 15, 5534–5540 (2009).
Stamme, C. et al. Temporal sequence of pulmonary and systemic inflammatory responses to graded polymicrobial peritonitis in mice. Infect. Immun. 67, 5642–5650 (1999).
Dugernier, T. L. et al. Compartmentalization of the inflammatory response during acute pancreatitis: correlation with local and systemic complications. Am. J. Respir. Crit. Care Med. 168, 148–157 (2003).
Lutgendorf, S. K. et al. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J. Clin. Oncol. 26, 4820–4827 (2008).
Sheehan, T. J., Fifield, J., Reisine, S. & Tennen, H. The measurement structure of the Center for Epidemiologic Studies Depression Scale. J. Pers. Assess. 64, 507–521 (1995).
Korenromp, I. H. et al. Reduced Th2 cytokine production by sarcoidosis patients in clinical remission with chronic fatigue. Brain Behav. Immun. 25, 1498–1502 (2011).
Langhorst, J. et al. Stress-related peripheral neuroendocrine-immune interactions in women with ulcerative colitis. Psychoneuroendocrinology 32, 1086–1096 (2007).
Capuron, L. & Miller, A. H. Cytokines and psychopathology: lessons from interferon-α. Biol. Psychiatry 56, 819–824 (2004).
Seigers, R. & Fardell, J. E. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci. Biobehav. Rev. 35, 729–741 (2011).
Pyter, L. M. et al. Mammary tumors induce select cognitive impairments. Brain Behav. Immun. 24, 903–907 (2010).
Krishnan, V. & Nestler, E. J. Animal models of depression: molecular perspectives. Curr. Top. Behav. Neurosci. 7, 121–147 (2011).
Pyter, L. M., Pineros, V., Galang, J. A., McClintock, M. K. & Prendergast, B. J. Peripheral tumors induce depressive-like behaviors and cytokine production and alter hypothalamic-pituitary-adrenal axis regulation. Proc. Natl Acad Sci. USA 106, 9069–9074 (2009).
Lamkin, D. M. et al. Cancer induces inflammation and depressive-like behavior in the mouse: modulation by social housing. Brain Behav. Immun. 25, 555–564 (2010).
Ray, M. A., Trammell, R. A., Verhulst, S., Ran, S. & Toth, L. A. Development of a mouse model for assessing fatigue during chemotherapy. Comp. Med. 61, 119–130 (2011).
Malik, N. M. et al. Behavioural and hypothalamic molecular effects of the anti-cancer agent cisplatin in the rat: A model of chemotherapy-related malaise? Pharmacol. Biochem. Behav. 83, 9–20 (2006).
Konat, G. W., Kraszpulski, M., James, I., Zhang, H. T. & Abraham, J. Cognitive dysfunction induced by chronic administration of common cancer chemotherapeutics in rats. Metab. Brain Dis. 23, 325–333 (2008).
Wood, L. J. et al. The cancer chemotherapy drug etoposide (VP-16) induces proinflammatory cytokine production and sickness behavior-like symptoms in a mouse model of cancer chemotherapy-related symptoms. Biol. Res. Nurs. 8, 157–169 (2006).
Seigers, R. et al. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav. Brain Res. 186, 168–175 (2008).
Gandal, M. J., Ehrlichman, R. S., Rudnick, N. D. & Siegel, S. J. A novel electrophysiological model of chemotherapy-induced cognitive impairments in mice. Neuroscience 157, 95–104 (2008).
Fardell, J. E., Vardy, J., Logge, W. & Johnston, I. Single high dose treatment with methotrexate causes long-lasting cognitive dysfunction in laboratory rodents. Pharmacol. Biochem. Behav. 97, 333–339 (2010).
Seigers, R. & Fardell, J. E. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci. Biobehav. Rev. 35, 729–741 (2010).
Malik, N. M., Liu, Y. L., Cole, N., Sanger, G. J. & Andrews, P. L. Differential effects of dexamethasone, ondansetron and a tachykinin NK1 receptor antagonist (GR205171) on cisplatin-induced changes in behaviour, food intake, pica and gastric function in rats. Eur. J. Pharmacol. 555, 164–173 (2007).
Acharya, M. M. et al. Human neural stem cell transplantation ameliorates radiation-induced cognitive dysfunction. Cancer Res. 71, 4834–4845 (2011).
Boyette-Davis, J., Xin, W., Zhang, H. & Dougherty, P. M. Intraepidermal nerve fiber loss corresponds to the development of taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain 152, 308–313 (2011).
Crawley, J. N. Behavioral phenotyping strategies for mutant mice. Neuron 57, 809–818 (2008).
Dantzer, R., Bluthe, R. M. & LeMoal, M. Experimental assessment of drug-induced changes in cognitive function: vasopressin as a case study. Neurotoxicology 9, 471–477 (1988).
Ward, R. D., Simpson, E. H., Kandel, E. R. & Balsam, P. D. Modeling motivational deficits in mouse models of schizophrenia: behavior analysis as a guide for neuroscience. Behav. Processes 87, 149–156 (2011).
Krishnan, V. & Nestler, E. J. The molecular neurobiology of depression. Nature 455, 894–902 (2008).
Carmichael, M. D. et al. Role of brain IL-1β on fatigue after exercise-induced muscle damage. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1344–R1348 (2006).
Carmichael, M. D. et al. Role of brain macrophages on IL-1beta and fatigue following eccentric exercise-induced muscle damage. Brain Behav. Immun. 24, 564–568 (2010).
Zemke, D. & Majid, A. The potential of minocycline for neuroprotection in human neurologic disease. Clin. Neuropharmacol. 27, 293–298 (2004).
O'Connor, J. C. et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2, 3-dioxygenase activation in mice. Mol. Psychiatry 14, 511–522 (2009).
Boyette-Davis, J. & Dougherty, P. M. Protection against oxaliplatin-induced mechanical hyperalgesia and intraepidermal nerve fiber loss by minocycline. Exp. Neurol. 229, 353–357 (2011).
Willemen, H. L. et al. Microglial/macrophage GRK2 determines duration of peripheral IL-1β-induced hyperalgesia: contribution of spinal cord CX3CR1, p38 and IL-1 signaling. Pain 150, 550–560 (2010).
Zink, M. C. et al. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA 293, 2003–2011 (2005).
Sacktor, N. et al. Minocycline treatment for HIV-associated cognitive impairment: results from a randomized trial. Neurology 77, 1135–1142 (2011).
Ho, E. L. et al. Minocycline fails to modulate cerebrospinal fluid HIV infection or immune activation in chronic untreated HIV-1 infection: results of a pilot study. AIDS Res. Ther. 8, 17 (2011).
Buch, M. H. & Emery, P. New therapies in the management of rheumatoid arthritis. Curr. Opin. Rheumatol. 23, 245–251 (2011).
Numerof, R. P. & Asadullah, K. Cytokine and anti-cytokine therapies for psoriasis and atopic dermatitis. BioDrugs 20, 93–103 (2006).
Monk, J. P. et al. Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J. Clin. Oncol. 24, 1852–1859 (2006).
Penna, F. et al. Anti-cytokine strategies for the treatment of cancer-related anorexia and cachexia. Expert Opin. Biol. Ther. 10, 1241–1250 (2010).
Jatoi, A. et al. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9). Lung Cancer 68, 234–239 (2009).
Goedendorp, M. M., Gielissen, M. F., Verhagen, C. A. & Bleijenberg, G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database of Systematic Reviews, Issue 1. Art. No.: CD006953. http://dx.doi.org/10.1002/14651858.CD006953.
Payne, C., Wiffen, P. J. & Martin, S. Interventions for fatigue and weight loss in adults with advanced progressive illness. Cochrane Database of Systematic Reviews, Issue 1. Art. No.: CD008427. http://dx.doi.org/10.1002/14651858.CD008427.pub2.
Brell, J. M. & Minasian, L. M. in Cancer Symptom Science: Measurement and Management (eds Cleeland, C. S., Fisch, M. J. & Dunn, A. J.) 315–324 (Cambridge University Press, Cambridge, 2011).
Cleeland, C. S., Fisch, M. J. & Dunn, A. J. in Cancer Symptom Science: Measurement and Management (eds Cleeland, C. S., Fisch, M. J. & Dunn, A. J.) 341–348 (Cambridge University Press, Cambridge, 2011).
Dantzer, R., O'Connor, J. C., Freund, G. G., Johnson, R. W. & Kelley, K. W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56 (2008).
Capuron, L. & Miller, A. H. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol. Ther. 130, 226–238 (2011).
Cavaletti, G., Alberti, P. & Marmiroli, P. Chemotherapy-induced peripheral neurotoxicity in the era of pharmacogenomics. Lancet Oncol. 12, 1151–1161 (2011).
Bull, S. J. et al. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Mol. Psychiatry 14, 1095–1104 (2009).
Aouizerat, B. E. et al. Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biol. Res. Nurs. 11, 27–41 (2009).
Miaskowski, C. et al. Preliminary evidence of an association between a functional interleukin-6 polymorphism and fatigue and sleep disturbance in oncology patients and their family caregivers. J. Pain Symptom Manage. 40, 531–544 (2010).
Froklage, F. E., Reijneveld, J. C. & Heimans, J. J. Central neurotoxicity in cancer chemotherapy: pharmacogenetic insights. Pharmacogenomics 12, 379–395 (2011).
Ahles, T. A. & Saykin, A. J. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat. Rev. Cancer 7, 192–201 (2007).
Cole, S. W. Elevating the perspective on human stress genomics. Psychoneuroendocrinology 35, 955–962 (2010).
Miller, G. E. et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc. Natl Acad. Sci. USA 106, 14716–14721 (2009).
Greene, W. C. & Chen, L. F. Regulation of NF-κB action by reversible acetylation. Novartis Found. Symp. 259, 208–217 (2004).
Lee, H. et al. Persistently activated Stat3 maintains constitutive NF-κB activity in tumors. Cancer Cell 15, 283–293 (2009).
Capuron, L. et al. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol. Psychiatry 70, 175–182 (2011).
Salamone, J. D., Correa, M., Farrar, A. & Mingote, S. M. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology 191, 461–482 (2007).
Larson, S. J., Romanoff, R. L., Dunn, A. J. & Glowa, J. R. Effects of interleukin-1beta on food-maintained behavior in the mouse. Brain Behav. Immun. 16, 398–410 (2002).
Miaskowski, C., Aouizerat, B. E., Dodd, M. & Cooper, B. Conceptual issues in symptom clusters research and their implications for quality-of-life assessment in patients with cancer. J. Natl Cancer Inst. Monogr. 2007, 39–46 (2007).
Wang, X. S. et al. Impact of cultural and linguistic factors on symptom reporting by patients with cancer. J. Natl Cancer Inst. 102, 732–738 (2010).
Barsevick, A. M. The elusive concept of the symptom cluster. Oncol. Nurs. Forum 34, 971–980 (2007).
Kirkova, J., Walsh, D., Aktas, A. & Davis, M. P. Cancer symptom clusters: old concept but new data. Am. J. Hosp. Palliat. Care 27, 282–288 (2010).
Aktas, A., Walsh, D. & Rybicki, L. Symptom clusters: myth or reality? Palliat. Med. 24, 373–385 (2010).
Fan, G., Filipczak, L. & Chow, E. Symptom clusters in cancer patients: a review of the literature. Curr. Oncol. 14, 173–179 (2007).
Imler, J. L. & Hoffmann, J. A. Toll receptors in innate immunity. Trends Cell Biol. 11, 304–311 (2001).
Sims, G. P., Rowe, D. C., Rietdijk, S. T., Herbst, R. & Coyle, A. J. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 28, 367–388 (2010).
Konsman, J. P., Parnet, P. & Dantzer, R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 25, 154–159 (2002).
Gaykema, R. P. & Goehler, L. E. Ascending caudal medullary catecholamine pathways drive sickness-induced deficits in exploratory behavior: brain substrates for fatigue? Brain Behav. Immun. 25, 443–460 (2011).
Konsman, J. P. et al. Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur. J. Neurosci. 28, 2499–2510 (2008).
Stone, E. A., Lehmann, M. L., Lin, Y. & Quartermain, D. Depressive behavior in mice due to immune stimulation is accompanied by reduced neural activity in brain regions involved in positively motivated behavior. Biol. Psychiatry 60, 803–811 (2006).
Grossberg, A. J. et al. Inflammation-induced lethargy is mediated by suppression of orexin neuron activity. J. Neurosci. 31, 11376–11386 (2011).
Huang, A. et al. Serum tryptophan decrease correlates with immune activation and impaired quality of life in colorectal cancer. Br. J. Cancer 86, 1691–1696 (2002).
Capuron, L. et al. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol. Psychiatry 54, 906–914 (2003).
O'Connor, J. C. et al. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2, 3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J. Neurosci. 29, 4200–4209 (2009).
Meagher, M. W. in Cancer Symptom Science: Measurement and Management (eds Cleeland, C. S., Fisch, M. J. & Dunn, A. J.) 124–141 (Cambridge University Press, Cambridge, 2011).
Berridge, K. C. & Robinson, T. E. Parsing reward. Trends Neurosci. 26, 507–513 (2003).
Bluthe, R. M., Dantzer, R. & Le Moal, M. Peripheral injections of vasopressin control behavior by way of interoceptive signals for hypertension. Behav. Brain Res. 18, 31–39 (1985).
Aubert, A. & Dantzer, R. The taste of sickness: lipopolysaccharide-induced finickiness in rats. Physiol. Behav. 84, 437–444 (2005).
Acknowledgements
The research described was supported by Award Numbers R01 MH079829 and R01 NS073939 to R. Dantzer, and P01 CA124787 and R01 CA026582 to C. S. Cleeland, and R01-NS060822 to M. W. Meagher. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to researching data for the article, discussing content, and writing and editing the manuscript prior to submission, and revising the article after peer review.
Corresponding author
Ethics declarations
Competing interests
R. Dantzer has received recent honoraria from Lundbeck Laboratories and Janssen. He is also a consultant for Lundbeck Laboratories. C. S. Cleeland has been a consultant/advisor for Abbott, Amgen, BMS-Bristol Myers Squibb, Exelixis, Genentech, and Pfizer. C. S. Cleeland has also received research funds from AstraZeneca. M. W. Meagher declares no competing interests.
Rights and permissions
About this article
Cite this article
Dantzer, R., Meagher, M. & Cleeland, C. Translational approaches to treatment-induced symptoms in cancer patients. Nat Rev Clin Oncol 9, 414–426 (2012). https://doi.org/10.1038/nrclinonc.2012.88
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2012.88
This article is cited by
-
The effect of treatment and coping on the quality of life in breast cancer patients: a moderated mediation model
Quality of Life Research (2022)
-
Symptom science research conducted in community programs funded by the US National Cancer Institute: a 12-year review, 2008 to 2019
Supportive Care in Cancer (2022)
-
MOFs-based nanoagent enables dual mitochondrial damage in synergistic antitumor therapy via oxidative stress and calcium overload
Nature Communications (2021)
-
Music, heart rate variability, and symptom clusters: a comparative study
Supportive Care in Cancer (2020)
-
Minocycline for symptom reduction during radiation therapy for head and neck cancer: a randomized clinical trial
Supportive Care in Cancer (2020)