Abstract
Adolescence is a vulnerable period for the development of substance use and related problems. Understanding how exposure to drugs influences the adolescent brain could reveal mechanisms underlying risk for addiction later in life. In the current study, 87 adolescents (16–20-year olds; the local legal drinking age was16, allowing the inclusion of younger subjects than usually possible) underwent EEG measurements during a Go/No-Go task with and without alcohol cues; after placebo and a low dose of alcohol (0.45 g/kg). Conflict monitoring and error detection processes were investigated with the N2 and the error-related negativity (ERN) ERP components. Participants were followed-up after 6 months to assess changes in alcohol use. The NoGo-N2 was larger for alcohol cues and acute alcohol decreased the amplitude of the NoGo-N2 for alcohol cues. ERN amplitude was blunted for alcohol cues. Acute alcohol decreased the amplitude of the ERN, specifically for control cues. Furthermore, the differences in ERN for alcohol cues between the placebo and alcohol conditions predicted alcohol use 6 months later: subjects who showed stronger blunting of the ERN after acute alcohol were more likely to return to more moderate drinking patterns. These results suggest that cues signalling reward opportunities might activate a go-response mode and larger N2 (detection of increased conflict) for these cues might be necessary for inhibition. The ERN results suggest a deficiency in the monitoring system for alcohol cues. Finally, a lack of alcohol-induced deterioration of error monitoring for cues with high salience might be a vulnerability factor for alcohol abuse in adolescents.
Similar content being viewed by others
INTRODUCTION
Dual process models explain addiction as the result of an imbalance between an appetitive and a regulatory system (Stacy et al, 2004; Deutsch et al, 2006; Wiers et al, 2007; but see Gladwin et al, 2011). Accordingly, poor response inhibition predicts drinking problems in high-risk children (Nigg et al, 2004; 2006) and a transition to problem drinking in adolescents (Norman et al, 2011). This may be related to the more general finding that adolescent cognitive performance is relatively weak in ‘hot’ (emotionally or motivationally salient) vs ‘cold’ contexts (Crone and Dahl, 2012; Grose-Fifer et al, 2013; Gladwin and Figner, 2014). Relatively weak performance of adolescents in an affective context has been tentatively related to a delay in the development of neural system needed for behavioural regulation, relative to the development of emotional-motivational system (Jentsch and Taylor, 1999; Casey and Jones, 2010). The anterior cingulate cortex (ACC) is a key structure involved in response inhibition and monitoring of response conflicts (co-activation of competing actions) (Yeung et al, 2004; Bekker et al, 2005). Given its rich connections to the prefrontal cortex and limbic structures, ACC-regulated processes are likely to be affected by the interplay between control and motivation. Neural activity associated with conflict monitoring has been associated with alcohol use severity (Claus et al, 2013) and density of family history of alcoholism (Fein and Chang, 2008). Thus, inhibition and conflict monitoring in an affective context are likely to play a role in the vulnerability for addiction in adolescents.
The acute disinhibiting effects of alcohol may lead to escalation of alcohol use (review: Field et al, 2010). This may be due to an increase in appetitive motivation towards drug cues and/or a decrease in regulatory cognitive control (Ridderinkhof et al, 2002; Duka and Townshend, 2004; Hernández and Vogel-Sprott, 2010; Adams et al, 2013). Acute alcohol effects may mimic long-term effects and could thus predict escalation (Wiers et al, 2007). Note that both relatively strong direct appetitive effects and relatively weak later responses to alcohol in terms of negative effects (eg, on balance) have been related to individual differences in the risk for later addiction (Newlin and Thomson, 1990; Schuckit et al, 2000).
The electroencephalogram (EEG) can be used to further study conflict monitoring, response inhibition, and error detection. According to the conflict monitoring theory, the ACC monitors conflict that arises because of co-activation of competing actions to deploy additional cognitive resources. The ACC generates the N2 event-related potential (ERP) component, when it detects pre-response conflict on correctly inhibited trials (Van Veen and Carter, 2002). Another ERP component, the error-related negativity (ERN), is thought to be related to error detection and generated by the ACC when a correct response is activated after an error, resulting in post-response conflict. Evidence supports the involvement of both conflict monitoring and response inhibition in N2 generation: the N2 was enhanced for low-frequency stimuli regardless whether a response must be generated or suppressed (Nieuwenhuis et al, 2003) and for NoGo stimuli when the frequency of required Go/NoGo responses was equal, also the response conflict (Lavric et al, 2004).
In adults, effects of alcohol on the ERP suggest impaired error detection but intact conflict monitoring (Ridderinkhof et al, 2002; Easdon et al, 2005; Bartholow et al, 2012). In a simulation study, Yeung and Cohen (2006) showed that the ERN and the N2 could be sensitive to relevant and irrelevant stimulus information, respectively. Further, the ERN is modulated by affective cues (Larson et al, 2006), which may be related to the disruption of inhibition in an affective context (Noël et al, 2007; Grose-Fifer et al, 2013). To our knowledge, acute alcohol effects on conflict monitoring in the context of motivationally relevant alcohol cues has not been investigated yet in drinking adolescents.
The current study focused on two questions: (i) Are response inhibition and conflict monitoring processes influenced by acute alcohol in adolescents and is this moderated by the motivational relevance of the cues? (ii) Do brain potentials, moderated by alcohol, predict future alcohol use in adolescents? To this aim, a Go/NoGo task including both alcohol and soft drink stimuli was used. We expected the ERN and the N2 to be dampened after acute alcohol and for alcohol vs soft drink cues. Participants’ change in alcohol use was assessed after 6 months. Differences across dose conditions were expected to predict short-term prospective escalation of alcohol use.
MATERIALS AND METHODS
Participants
Ninety-seven adolescents were recruited from local high schools via advertisements. Participants were required to be minimally 16 years old (minimum drinking age in Netherlands at the time of the study), with a minimum weight of 50 kg and to have had at least one full drink in their lifetime (see Supplementary Materials for exclusion criteria). Prior to the experiment, a written informed consent was obtained from all participants and from parents of participants under the age of 18. Ten subjects’ data were excluded for the following reasons: three because of positive drug test for THC, one because of a drop-out in the second session, four because of equipment failure, one because of incorrect beverage administration, and one because of an extreme number of omission trials. The analysis was conducted with the remaining 87 subjects (33 males, mean age=17.6 years, range=16–20 years).
Alcohol Administration and Procedure
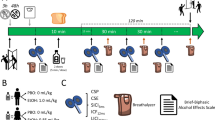
The study consisted of two sessions on two different days. On each session, either a placebo or an alcoholic drink (0.45 ml/kg) was administered. Beverages were divided into three equal portions. Two of the drinks were served prior to commencing the tasks and the last drink was administered as a booster drink halfway through the session (for details on the alcohol administration see Supplementary Materials). Upon arrival in the lab, subjects filled out demographics, questionnaires related to personality and drinking habits. At the start of each session, subjects completed the Desire for Alcohol Questionnaire (DAQ, Love et al, 1998) and the Positive and Negative Affect Scale (PANAS, Watson et al, 1988) to control for current mood and craving. Current alcohol use and problems were assessed with the Alcohol Use Disorder Identification Test (AUDIT, Saunders et al, 1993; we used both the standard past year version and a version about the past 3 months), the Ruthers Alcohol Problem Index (RAPI, White and Labouvie, 1989), and an adjusted version of the Timeline Followback method developed by Sobell and Sobell, (1992) as reported in Wiers et al (1997). Drug use behaviour was assessed with an 11-item rating scale (Graham et al, 1984). To assess drinking frequency separately for weekdays and weekends, subjects filled out three additional questions. This additional set included questions on the frequency and the quantity of drinking in the last 3 months and lifetime binge drinking frequency (see Supplementary Materials). The session started with an unrelated task, followed by beverage administration. Approximately 10 min after beverage administration, subjects also performed three unrelated tasks (see Supplementary Materials). Order of the tasks was counterbalanced across subjects, but was kept the same across sessions for each subject. Breath alcohol concentration (BrAC) was collected 5 min after the first two drinks, before and after the booster drink, and at the end of the experiment by using the Lion alcolmeter SD-400 (Lion Laboratories Limited, South Glamorgan, Wales). Participants filled out the Brief Biphasic Alcohol Effects Scale (B-BAES, Rueger et al, 2009) each time a breath sample was taken, except before the booster drink.
The sessions were carried out at least 48 h and maximally 1 week apart. Sessions started between 12 : 00 and 6 : 00 PM. Each session took approximately two and a half hours, including breaks and the application of electrodes. Six months after the baseline assessment, participants were contacted via email for an online assessment on recent alcohol and drug use. During the follow-up assessment, subjects filled out the same alcohol-related scales as during pre-test. The study was approved by the local ethical committee.
Go/NoGo Task
Subjects were presented with pictures of beverages in a bottle or in a glass. The task consisted of blocks with sets of either alcohol or soft drink pictures. In the alcohol blocks, the stimuli were alcohol-related pictures (eg, beer, wine), and in the neutral blocks, the stimuli were pictures of soft drinks (eg, cola, sprite). In the Go trials, a right button response was required for the pictures of a beverage in a bottle (as quick as possible while maintaining accurate). In the NoGo trials, when the picture of a beverage in a glass was presented, subjects were required to withhold their responses. Four pictures were used in each block type, each consisting of three go stimuli and one no-go stimulus (eg, beer, Figure 1). Each stimulus was presented with equal frequency, leading to 25% no-go and 75% go trials (90 no-go and 270 go trials per block). Pictures with and without alcohol contents were matched for perceptual characteristics (ie, colour, shape etc.).
Schematic representation of the alcohol-related Go/NoGo Task. Subjects were presented with pictures of beverages in a bottle or in a glass. The task consisted of blocks with sets of either alcohol or soft drink pictures. In the Go trials, a right button response was required for the pictures of a beverage in a bottle. In the NoGo trials, when the picture of a beverage in a glass was presented, subjects were required to withhold their responses. The task consisted of 10 blocks, with neutral and alcohol blocks alternating. Each stimulus was presented for 200 ms, followed by 800 ms and 1000 ms of ITI for go and no-go trials, respectively.
The task started either with the alcohol or the neutral block, counterbalanced across participants. After12 practice trials, the task consisted of 10 assessment blocks, with neutral and alcohol blocks alternating. Each stimulus was presented for 200 ms, followed by 800 ms and 1000 ms of ITI for go and no-go trials, respectively. To study error-related EEG activity, an adequate amount of commission errors were required, therefore an adaptive procedure was used. After each block, subjects’ overall commission errors and correct responses in the no-go trials were calculated. When the ratio between commission errors/correct no-go responses was smaller or larger than 50/50, the next block started with a feedback encouraging, respectively, to ‘speed up’ or ‘slow down’ the response. If the ratio was equal, subjects received no feedback. To control for the effect of picture familiarity across sessions, two sets of pictures with and without alcohol contents were matched, and each stimulus set was randomly assigned to a session.
EEG Recording and Data Analysis
Electrophysiological data were recorded from the scalp using an Active-Two amplifier (Biosemi, Amsterdam, The Netherlands) from 32-scalp sites. Electrodes were placed at the standard positions of the 10–20 international system. Two electrodes were placed at the outer canthi of the eyes to measure horizontal eye movements. Two electrodes were placed at below and above the left eye to measure vertical eye movements. EEG was recorded at 2048 Hz sampling rate. The distance between the screen and the subject was kept 75 cm.
EEG analysis was conducted using Brain Vision Analyzer (version 2.0, Brain Products GmbH, Munich, Germany). Data were down-sampled to 250 Hz, re-referenced offline to the average of scalp electrodes, low pass filtered at 20 Hz, and high pass filtered at 0.1 Hz. Ocular correction was applied using the algorithm of Gratton et al, (1983). Stimulus and response-locked epochs ranged from −200 to 1000 ms and from −300 to 800 ms, respectively. Trials were considered artifacts when the difference between consecutive data points was larger than 50 mV and the difference between the lowest and the highest voltage within a segment was higher than 150 mV. Epochs with an amplitude exceeding ±100 mV were excluded. The mean 200 ms pre-stimulus and pre-response period was used as baseline. After baseline correction, average stimulus-locked ERPs were calculated for artifact-free trials at each scalp location for trials with correct go, correct no-go, and commission responses separately. Average response-locked ERPs were created for trials with commission error responses only (for details on ERP quantification and subject/trial exclusion procedure, see Supplementary Materials).
Data Preparation and Statistical Analysis
For behavioral performance, mean RTs for correct go and commission error responses, average hit rates (trials with correct go response/trials with correct go plus omission responses), and false alarm rates (trials with commission response/trials with commission plus correct no-go responses) were calculated separately for the blocks with neutral and alcohol stimulus set in each condition.
All analyses were conducted using a repeated measures analysis of variance. The PANAS and DAQ scores were analyzed with Dose (Placebo, Alcohol) as a within-subject factor. The Stimulation and Sedation subscales of B-BAES scores were separately analyzed with Dose (Placebo, Alcohol), and Time (pre-task and post-task) as within-subject factors. The BrAC were subjected to a repeated measures analysis of variance, with Time (BrAC pre-task and post-task) as within-subject variable. Two subjects’ B-BAES data and five subjects’ BrAC scores were lost; the analysis was conducted with the remaining subjects. Behavioral data were analyzed with Dose (Placebo, Alcohol) and Beverage Image Class (Neutral, Alcohol Beverage Images) as within-subject factors. ERP data were analyzed with Dose (Placebo, Alcohol) and Beverage Image Class (Neutral, Alcohol Beverage Images) as within-subject variables. Further analysis for each ERP component focused on the channel locations where the amplitude was maximal. When appropriate, Greenhouse-Geisser corrected values were reported.
To assess whether ERP differences across sessions predicted unique variance in the change in alcohol use during the 6 months after the experiment, a hierarchical multiple regression analysis was conducted. First, subjects’ demographic characteristics (age, gender, and education) were entered to the regression model, followed by the AUDIT score for recent use (sum of scores of items on frequency of drinking, typical quantity, and frequency of heavy drinking) at baseline from the version about the past 90 days. In the last step, the contrast scores (alcohol minus placebo) for the alcohol and the neutral stimulus sets were entered. This way the predictive value of acute alcohol effect on ERP indices was tested beyond the predictive value of subjects’ demographics and AUDIT scores at baseline.
RESULTS
Questionnaires
Subjects’ craving scores and their positive and negative mood scores at the start of the experiment were the same in the placebo and in the alcohol condition (p-values>0.2).
Manipulation Checks
The differences in the BAES stimulation subscale before and after the task performance revealed that subjects felt less stimulated as the session proceeded (F(1, 84)=14.01, p<0.001, η2p=0.14). Moreover, subjects felt more sedated after alcohol than after placebo (F(1, 84)=29.84, p<0.001, η2p=0.26). BAL levels were lower post-task compared with pre-task (F(1, 81)=4.519, p=0.037, η2p=0.05; see Table 1).
Behavioral Measures
Dose by beverage image class effect on hit rates
Hit rates trended towards a main effect of Dose (F(1, 86)=3.89, p=0.052, η2p=0.04), an effect superseded by a significant interaction effect of Dose by Beverage Image Class (F(1, 86)=7.85, p=0.006, η2p=0.08). On the average, hit-rates tended to be higher in the placebo condition. Post hoc analysis of the two-way interaction revealed that in the alcohol condition, hit-rates were higher for the Alcohol Beverage Images compared with the Neutral Beverage Images (t(85)=−2.39, p=0.02), in the absence of such an effect in the placebo condition (p>0.15). Moreover, hit-rates were higher for the Neutral Beverage Images in the placebo condition compared with the alcohol condition (t(85)=3.09, p=0.003), with no differences observed between conditions for the Alcohol Beverage Images (p>0.55) (see Figure 2, left panel).
Behavioral results for hit rates (left side), false alarm rates (middle), and RT (right side). Hit rates were lower in alcohol condition for neutral beverage images. False alarm rates were lower for neutral beverage images. RTs for trials with commission errors and correct Go responses were shorter in the placebo condition. Alc, alcohol; Pla, placebo; Pla Correct, Correct go trials in the placebo condition; *p<0.05, **p<0.01, ***p<0.005, ****p<0.001.
Effect of beverage image class on false alarms
False alarm rates revealed a main effect of Beverage Image Type (F(1, 86)=32, p<0.001, η2p=0.27), subjects made more commission errors for the Alcohol than the Neutral Beverage Images (see Figure 2, middle panel).
Effect of dose on RT
In the trials with correct-go and commission responses, subjects tended to respond faster in the placebo than the alcohol condition (Correct-Go: F(1, 86)=3.56 p=0.063, η2p=0.04; Commission: F(1, 86)=5.91, p=0.017, η2p=0.06; See Figure 2, right panel).
N2
Dose by beverage image class effect on N2
The NoGo-N2 for the correct responses revealed a main effect of Dose (F(1, 77)=10.103, p=0.002, η2p=0.12) and a main effect of Beverage Image Class (F(1, 77)=24.888, p<0.001, η2p=0.24). An interaction effect of Block Type by Beverage Image Class qualified these main effects (F(1, 77)=6.021, p=0.016, η2p=0.073). Inspection of the two-way interaction effect revealed that: (i) The NoGo-N2 for the Alcohol Beverage Images was larger than for the Neutral Beverage Images, both in the placebo and in the alcohol conditions (placebo: t(77)=5.47, p<0.001, alcohol: t(77)=2.02, p=0.047); (ii) For the Neutral Beverage Images, the NoGo-N2 had comparable amplitudes (p>0.2) after alcohol and placebo, however, for the Alcohol Beverage Images, acute alcohol decreased the amplitude of NoGo-N2, (t(77)=−4.136, p<0.001) (see Figure 3, left). NoGo-N2 for the incorrect trials did not reveal any main or interaction effects (p-values >0.07).
Stimulus-locked N2 (left side) for trials with correct responses and response-locked ERN for trials with error responses (right side) at Fz. Stimulus and response onset occurred at 0 ms. The NoGo-N2 for the Alcohol Beverage Images was larger than the Neutral Beverage Images in both placebo and alcohol conditions and acute alcohol decreased the amplitude of NoGo-N2 only for the Alcohol Beverage Images (left side). The ERN was smaller in the alcohol than the placebo condition. Acute alcohol decreased the ERN only for the Neutral Beverage Images (right side). Alc. Bev. Ima, alcohol beverage images; Neu. Bev. Ima, neutral beverage images; *p<0.05, **p<0.01, ***p<0.005, ****p<0.001.
Error-Related Negativity
Dose by beverage image class effect on ERN
The ERN was smaller in the alcohol than the placebo condition (F(1, 68)=4.073, p=0.048, η2p=0.057) (see Figure 3, right panel). Given the predictive effects of the ERN (see below), additional exploratory pair-wise comparisons were conducted. These results revealed that acute alcohol decreased the ERN for the Neutral Beverage Images (t(68)=−2.22, p=0.03), but not for the Alcohol Beverage Images (p>0.3).
Neural Predictors of Alcohol Use After Six Months
Six months after the baseline assessment, 82.5% follow-up response rate was achieved in the full sample. On average, ‘completers’ and ‘drop-outs’ were similar on demographic characteristics, yet drop-outs scored higher on drinking-related problems (RAPI), contained more smokers and reported higher drug use frequency (see Supplementary Table S2). In the hierarchical multiple regression model, one subject’s Cook’s distance was 0.7 (mean Cook’s Distance=0.01, SD Cook’s Distance=0.03, before exclusion) and this subject was excluded from the analysis. The frontal ERN in the Alcohol Beverage Images significantly predicted future alcohol use beyond the variance explained by demographics and baseline AUDIT scores. The total variance explained by the full model was 69% (F change2,51=5.886, p=0.005). The demographics and the baseline AUDIT scores explained 19.3% (F change4,54=3.237, p=0.019) and 42.5% (F change1,53=58.875, p<0.001) of the variance in alcohol use 6 months later, respectively. The frontal ERN in the Alcohol Beverage Images explained an additional 7.2% of the variance. To interpret the contribution of the ERP contrast in the Alcohol Beverage Images, we conducted a correlation analysis between change in AUDIT scores (AUDIT follow-up—AUDIT baseline), the ERN contrast in the alcohol and neutral blocks. The results revealed a negative correlation between change in AUDIT and the ERN contrast in the Alcohol Beverage Images (r=−0.42, p=0.001) (see Figure 4). Subjects, who showed a relatively strong ERN decrease after acute alcohol in the alcohol blocks had lower AUDIT scores, relative to baseline, 6 months later.
Hierarchical multiple regression analysis for variables predicting AUDIT at 6-month follow-up (n=59) (left side). The correlation between change in AUDIT scores (AUDIT follow-up, last 90 days—AUDIT baseline, last 90 days), the ERN contrast (Alcohol—Placebo) for the Alcohol (upper, right) and Neutral Beverage Images (lower, right). AlcBevIma, alcohol beverage images; NeuBevIma, neutral beverage images; SE, Standard errors.
DISCUSSION
We examined whether acute alcohol and alcohol cues affect conflict monitoring and error detection processes in drinking older adolescents. Moreover, we tested whether alcohol-induced changes on these cognitive processes predict future alcohol use. Behavioral data revealed that RT for commission and correct-go responses were slower after alcohol administration, suggesting a psychomotor slowing to maintain accuracy. In line with this interpretation, false alarm rates did not vary across the alcohol and placebo conditions. Similar to previous findings (Adams et al, 2013; Kreusch et al, 2013), subjects gave more go responses for alcohol cues both in Go and NoGo trials, suggesting that alcohol cues may be more associated with an approach or a go response. Moreover, hit rates for neutral cues were more sensitive to acute alcohol effects; subjects made more omissions for neutral cues after acute alcohol.
The ERP data showed that the NoGo-N2 for alcohol cues was higher than for soft drink cues, suggesting a relatively strong simultaneous activation of Go (stimulus-induced) and NoGo (task-induced) responses towards alcohol cues. In line with previous simulation research (Yeung and Cohen, 2006), acute alcohol decreased the N2 specifically for task-irrelevant alcohol cues. The ERN was not influenced by the motivationally salient alcohol cues. Exploratory analyses revealed lower ERN amplitudes after acute alcohol, specifically for neutral cues. Finally, alcohol-induced changes in the ERN for alcohol cues predicted changes in alcohol use 6 months later.
In young adult drinkers, many studies have shown increased salience of alcohol-related stimuli (Herrmann et al, 2000; Bartholow et al, 2007; 2010), engagement of attentional resources, and automatic approach tendencies towards alcohol cues (Johnsen et al, 1994; Sharma et al, 2001; Field et al, 2008). Thus, the results of the Nogo-N2 associated with inhibition and conflict monitoring might indicate that alcohol cues pre-activate a go response because of increased attention allocation and approach tendencies. In correct NoGo trials, the greater N2 for alcohol cues might suggest that when increased conflict between stimulus-induced and task-relevant responses is detected, inhibition of this pre-potent response has been successful. In incorrect NoGo trials, the lack of this additional process of conflict detection might have resulted in comparable N2 amplitudes for alcohol and non-alcohol cues. Moreover, the ERN associated with error detection was smaller for alcohol cues during commission errors, a result in line with the idea that detecting the conflict between competing responses might be important for giving correct responses. Moreover, relatively small ERN amplitudes for alcohol cues might suggest a relative dysfunction involving error detection in the presence of alcohol cues.
To the best of our knowledge, only two previous studies investigated the influence of drug-related context on the N2, one in the context of smoking cues (Luijten et al, 2011) and the other in the context of alcohol cues (Petit et al, 2012). These studies did not reveal any effects of drug-related cues on the N2. Both studies implemented alcohol-related contexts as backgrounds; the feature that signaled the correct response was not itself drug-related. This may have allowed the drug-related stimuli to be more effectively suppressed. The study by Luijten et al (2011) implemented intermittent presentation of drug-related and control cues, unlike our continuous presentation of drug cues in a blocked design. Rapid attention alterations required by the task might have reduced the effect of task-irrelevant stimulus information on the N2. Differences across studies could also be because of studying different populations. The current study tested such effects with a younger sample, likely to have heightened reward sensitivity.
A second aim of the current study was to examine whether alcohol-induced changes on ERPs associated with action monitoring would predict changes in alcohol use. The effect of acute alcohol in the ERN for alcohol cues predicted changes in alcohol use in adolescents. Subjects for whom alcohol disrupted the error detection processes for alcohol cues, as indexed by the ERN, were more likely to show a decrease in their drinking at the 6-month follow-up. A tangible deleterious effect of acute alcohol on the ERN for alcohol cues might indicate a ‘protective sensitivity’, comparable with the protective value of negative alcohol effects on body sway (cf., Schuckit et al, 2000). An earlier study showed that expectancy of cognitive and motor impairment due to alcohol is associated with non-drinking in adolescents and young adults (Wiers et al, 1997). Moreover, individuals with high sensitivity to negative alcohol effects are more likely to show adaptive strategy adjustments (Bartholow et al, 2003). Taken together, alcohol-induced changes in the monitoring system might be a protective factor for alcohol abuse.
In summary, the results of the current study are in line with previous studies showing decreased performance in the presence of motivational cues. We showed that the conflict monitoring system is sensitive to alcohol cues. This could be because cues signaling reward opportunities might activate a go response mode. Future research is needed to replicate and extend the current findings in adults with substance use disorders. Moreover, low and high doses of alcohol affect different processes; therefore, future studies in adult populations could study the acute effects of higher dosages of alcohol and relate them to future alcohol use. Responses of the error detection system towards drugs and drug-related stimuli appear to be related to changes in drug-related behaviors. An interesting route for future studies would be to understand how sensitivity to positive and negative response outcomes (ie, feedback-based learning) could affect processes such as error detection and conflict monitoring in adolescents and how these learning processes could contribute to addictive behaviors in later life. Lastly, we would like to note that until now, our knowledge of acute alcohol effects on neurocognitive processes in younger samples have exclusively been based on either relatively old adolescents because of legal limitations or animal studies, hence the current study uniquely contributes to the literature by providing initial findings of acute alcohol effects on human adolescent population.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
References
Adams S, Ataya AF, Attwood AS, Munafo MR (2013). Effects of alcohol on disinhibition towards alcohol-related cues. Drug Alcohol Depend 127: 137–142.
Bartholow BD, Henry EA, Lust SA (2007). Effects of alcohol sensitivity on P3 event-related potential reactivity to alcohol cues. Psychol Addict Behav 21: 555–563.
Bartholow BD, Henry EA, Lust SA, Saults JS, Wood PK (2012). Alcohol effects on performance monitoring and adjustment: affect modulation and impairment of evaluative cognitive control. J Abnorm Psychol 121: 173–186.
Bartholow BD, Lust SA, Tragesser SL (2010). Specificity of P3 event-related potential reactivity to alcohol cues in individuals low in alcohol sensitivity. Psychol Addict Behav 24: 220–228.
Bartholow BD, Pearson M, Sher KJ, Wieman LC, Fabiani M, Gratton G (2003). Effects of alcohol consumption and alcohol susceptibility on cognition: a psychophysiological examination. Biol Psychol 64: 167–190.
Bekker EM, Kenemans JL, Verbaten MN (2005). Source analysis of the N2 in a cued Go/NoGo task. Brain Res Cogn Brain Res 22: 221–231.
Casey BJ, Jones RM (2010). Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J Am Acad Child Adolesc Psychiatry 49: 1189–1201.
Claus E, Feldstein Ewing SW, Filbey FM, Hutchison KE (2013). Behavioral control in alcohol use disorders: relationships with severity. J Stud Alcohol Drugs 74: 141–151.
Crone EA, Dahl RE (2012). Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci 13: 636–650.
Deutsch R, Gawronski B, Strack F (2006). At the boundaries of automaticity: negation as reflective operation. J Pers Soc Psychol 91: 385–405.
Duka T, Townshend JM (2004). The priming effect of alcohol pre-load on attentional bias to alcohol-related stimuli. Psychopharmacology (Berl) 176: 353–361.
Easdon C, Izenberg A, Armilio ML, Yu H, Alain C (2005). Alcohol consumption impairs stimulus- and error-related processing during a Go/No-Go Task. Brain Res Cogn Brain Res 25: 873–883.
Fein G, Chang M (2008). Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment-naïve alcoholics. Drug Alcohol Depend 92: 141–148.
Field M, Kierman A, Eastwood B, Child R (2008). Rapid approach responses to alcohol cues in heavy drinkers. J Behav Ther Exp Psy 39: 209–218.
Field M, Wiers RW, Christiansen P, Fillmore MT, Verster JC (2010). Acute alcohol effects on inhibitory control and implicit cognition: Implications for loss of control over drinking. Alcohol 34: 1346–1352.
Gladwin TE, Figner B (2014). "Hot" cognition and dual systems: Introduction, criticisms, and ways forward. In: Wilhelms E, Reyna VF (eds). Frontiers of Cognitive Psychology Series: Neuroeconomics, Judgment and Decision Making. Psychological Press: New York. pp 157–180.
Gladwin TE, Figner B, Crone EA, Wiers RW (2011). Addiction, adolescence, and the integration of control and motivation. Dev Cogn Neurosci 1: 364–376.
Graham JW, Flay BR, Johnson CA, Hansen WB, Grossman L, Sobel JL (1984). Reliability of self-report measures of drug use in prevention research: Evaluation of the Project SMART questionnaire via the test–retest reliability matrix. J Drug Edu 14: 175–193.
Gratton G, Coles MGH, Donchin E (1983). A new method for off-line removal of ocular artifact. Eletroencephalogr Clin Neurophysiol 55: 468–484.
Grose-Fifer J, Rodrigues A, Hoover S, Zottoli T (2013). Attentional capture by emotional faces in adolescence. Adv Cogn Psychol 9: 81–91.
Hernández OH, Vogel-Sprott M (2010). Alcohol slows the brain potential associated with cognitive reaction time to an omitted stimulus. J Stud Alcohol Drugs 71: 268–277.
Herrmann MJ, Weijers HG, Wiesbeck GA, Aranda D, Böning J, Fallgatter AJ (2000). Event-related potentials and cue-reactivity in alcoholism. Alcohol Clin Exp Res 24: 1724–1729.
Jentsch JD, Taylor JR (1999). Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 146: 373–390.
Johnsen BH, Laberg JC, Cox WM, Vaksdal A, Hugdahl K (1994). Alcoholic subjects ’ attentional bias in the processing of alcohol-related words. Psychol Addict Behav 8: 111–115.
Kreusch F, Vilenne A, Quertemont E (2013). Response inhibition towards alcohol-related cues using an alcohol go/no-go task in problem and non-problem drinkers. Addict Behav 38: 2520–2528.
Larson MJ, Perlstein WM, Stigge-Kaufman D, Kelly KG, Dotson VM (2006). Affective context-induced modulation of the error-related negativity. Neuroreport 17: 329–333.
Lavric A, Pizzagalli DA, Forstmeier S (2004). When ‘go’ and ‘nogo’ are equally frequent: ERP components and cortical tomography. Eur J Neurosci 20: 2483–2488.
Love A, Es DJAM, Willner P (1998). A comparison of two alcohol craving questionnaires. Addiction 93: 1091–1102.
Luijten M, Littel M, Franken IHA (2011). Deficits in inhibitory control in smokers during a Go/NoGo task: an investigation using event-related brain potentials. PLoS One 6: e18898.
Newlin DB, Thomson JB (1990). Alcohol challenge with sons of alcoholics: A critical review and analysis. Psychol Bull 108: 383–402.
Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR (2003). Electrophysiological correlates of anterior cingulate function in a go/nogo task: effects of response conflict and trial type frequency. Cogn Affect Behav Neurosci 3: 17–26.
Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitzgerald HE et al (2004). Neuropsychological executive functioning in children at elevated risk for alcoholism: findings in early adolescence. J Abnorm Psychol 113: 302–314.
Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM et al (2006). Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry 45: 468–475.
Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF (2011). Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend 119: 216–223.
Noël X, Linden M, Van der, d’Acremont M, Bechara A, Dan B, Hanak C et al (2007). Alcohol cues increase cognitive impulsivity in individuals with alcoholism. Psychopharmacology (Berl) 192: 291–298.
Petit G, Kornreich C, Noël X, Verbanck P, Campanella S (2012). Alcohol-related context modulates performance of social drinkers in a visual Go/No-Go task: a preliminary assessment of event-related potentials. PLoS One 7: e37466.
Ridderinkhof KR, De Vlugt Y, Bramlage A, Spaan M, Snel J, Band GPH (2002). Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science 298: 2209–2211.
Rueger SY, McNamara PJ, King AC (2009). Expanding the utility of the Biphasic Alcohol Effects Scale (BAES) and initial psychometric support for the Brief-BAES (B-BAES). Alcohol Clin Exp Res 33: 916–924.
Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction 88: 791–804.
Schuckit MA, Smith TL, Kalmijn J, Tsuang J, Hesselbrock V, Bucholz K (2000). Response to alcohol in daughters of alcoholics: a pilot study and a comparison with sons of alcoholics. Alcohol Alcohol 35: 242–248.
Sharma D, Albery IP, Cook C (2001). Selective attentional bias to alcohol related stimuli in problem drinkers and non-problem drinkers. Addiction 96: 285–295.
Sobell LC, Sobell MB (1992). Timeline followback: A technique for assessing self-reported alcohol consumption. In: Allen J, Litten RZ (eds). Measuring alcohol consumption: Psychosocial and biological methods. Humana: Totowa NJ. pp 41–72.
Stacy AW, Ames SL, Knowlton BJ (2004). Neurologically plausible distinctions in cognition relevant to drug use etiology and prevention. Subst Use Misuse 39: 1571–1623.
Van Veen V, Carter C (2002). The anterior cingulate as a conflict monitor: fMRI and ERP studies. Psychol Behav 77: 477–482.
Watson D, Clark LA, Tellegen A (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54: 1063–1070.
White HR, Labouvie EW (1989). Towards the assessment of adolescent drinking problems. J Stud Alcohol 50: 30–37.
Wiers RW, Bartholow BD, Wildenberg E, van den, Thush C, RCME Engels, Sher KJ et al (2007). Automatic and controlled processes and the development of addictive behaviors in adolescents: a review and a model. Pharmacol Biochem Behav 86: 263–283.
Wiers RW, Hoogeveen K, Sergeant JA, Gunning WB (1997). High- and low-dose alcohol-related expectancies and the differential associations with drinking in male and female adolescents and young adults. Addiction 92: 871–888.
Yeung N, Botvinick MM, Cohen JD (2004). The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychol Rev 111: 931–959.
Yeung N, Cohen JD (2006). The impact of cognitive deficits on conflict monitoring. Psychol Sci 17: 164–171.
Acknowledgements
The authors are supported by VICI award 453.08.01 from the Netherlands National Science Foundation (N.W.O.), awarded to the senior author. Thomas E. Gladwin is supported by ERAB grant EA 1239.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Korucuoglu, O., Gladwin, T. & Wiers, R. Alcohol-Induced Changes in Conflict Monitoring and Error Detection as Predictors of Alcohol Use in Late Adolescence. Neuropsychopharmacol 40, 614–621 (2015). https://doi.org/10.1038/npp.2014.209
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2014.209
This article is cited by
-
Acute alcohol does not impair attentional inhibition as measured with Stroop interference scores but impairs Stroop performance
Psychopharmacology (2021)
-
Performance on emotional tasks engaging cognitive control depends on emotional intelligence abilities: an ERP study
Scientific Reports (2017)
-
On the Development of Implicit and Control Processes in Relation to Substance Use in Adolescence
Current Addiction Reports (2015)