Abstract
Many children with childhood-onset obsessive–compulsive disorder (OCD) fail to respond adequately to standard therapies. Evidence from preclinical and clinical studies suggests that the glutamatergic neurotransmitter system might be an alternative treatment target. This study examined the efficacy of riluzole, a glutamatergic modulator, as an adjunctive therapy for children with treatment-resistant OCD. In a 12-week, double-blind, placebo-controlled study, 60 treatment-resistant children and adolescents (mean age=14.5±2.4 years), with moderate to severe OCD (mean Children’s Yale-Brown Obsessive–Compulsive Scale (CY-BOCS)=28.2±3.7), 17 of whom also had concomitant autism spectrum disorder, were randomized to receive riluzole (final dose of 100 mg/day) or placebo in addition to the existing treatment regimen. Fifty-nine subjects completed the randomized trial. Primary outcome measures were changes on the CY-BOCS, the Clinical Global Impressions Scale, and the Children’s Global Assessment Scale. Riluzole was fairly well tolerated, although it was associated with one case of pancreatitis and five instances of slight increases in transaminases. All subjects showed significant reductions in CY-BOCS scores during treatment; however, there was no significant difference between placebo and riluzole on any of the primary or secondary outcome measures. The study failed to demonstrate superiority of riluzole over placebo as an adjunctive treatment for children with childhood-onset OCD. However, future studies may show benefits for less treatment-refractory children with fewer concomitant medications.
Similar content being viewed by others
INTRODUCTION
Obsessive–compulsive disorder (OCD) is a common psychiatric illness in childhood, affecting 1–2% of youth worldwide (Geller, 2006). It often causes significant morbidity and persists across the lifespan (Micali et al, 2010). Established treatments for childhood OCD include the serotonin reuptake inhibitors (SRIs) and cognitive-behavioral therapy (CBT), particularly exposure and response prevention (Geller et al, 2003; Watson and Rees, 2008). Moreover, the combination of medication and CBT may be more effective than either treatment alone (Pediatric OCD Treatment Study, 2004). However, significant numbers of young people with OCD remain impaired despite these options (Stewart et al, 2004). Thus, there is a need for additional treatment options.
The neural basis for OCD is thought to involve circuits including the orbitofrontal cortex, striatum, and thalamus, and the neurotransmitters serotonin, dopamine, glutamate, and gamma-amino-butyric acid (MacMaster et al, 2008; Menzies et al, 2008). The evidence for targeting glutamate, in particular, comes from a number of directions, including imaging, genetic, animal models, and treatment studies (for review, Wu et al, 2012). For example, in a proton magnetic resonance spectroscopy study of pediatric subjects, abnormally elevated glutamate/glutamine concentrations in the caudate nucleus normalized among SRI responders (Rosenberg et al, 2000). In addition, reports of associations between OCD (in males) and a genetic locus that codes for an excitatory amino-acid transporter (which is important in terminating the action of glutamate in the synapse) suggest that glutamate excess may be relevant in the disorder (Arnold et al, 2006; Dickel et al, 2006).
Glutamate has been a target of drug development for a number of neurologic/psychiatric conditions (Javitt et al, 2011), including autistic disorder. Open-label case series of lamotrigine, amantadine, memantine, acamprosate, and D-cycloserine have reported anecdotal benefits, but very few controlled trials have been conducted. Controlled trials of dextromethorphan, lamotrigine, and amantadine found no effect on symptoms associated with autism spectrum disorder (ASD; for revew, Farmer et al, 2013). Aside from a letter detailing the successful treatment of three adolescents and adults with autism (Wink et al, 2011), there have been no reports of effects of riluzole on autistic symptoms nor on its utility in treating patients with co-occurring OCD and ASD.
Riluzole is considered a ‘glutamatergic modulator’ with effects on glutamate release and enhancement of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid trafficking and glial excitatory amino-acid transporters (Bellingham, 2011). Currently, the only FDA indication for riluzole is in amyotrophic lateral sclerosis; there are no indications for childhood conditions. Recently, riluzole has been studied in a number of open-label trials for generalized anxiety disorder, major depressive disorder, bipolar depression, and OCD in adults, as well as in one double-blind trial (see review: Zarate and Manji, 2008). These reports suggest beneficial effects of riluzole. Riluzole is generally well tolerated; it is occasionally associated with mild reversible elevations in transaminases (Zarate and Manji, 2008).
We previously conducted a small open-label trial of riluzole for childhood OCD, where four of six participants had significant improvement in OCD symptoms (Grant et al, 2007). Riluzole was well tolerated, with no limiting adverse effects. Based on the pilot data, we hypothesized that treatment with riluzole over the course of 12 weeks would result in greater improvements than placebo in obsessive–compulsive symptoms in children with treatment-resistant OCD. In addition, a subgroup of children with co-occurring ASD and OCD was enrolled to evaluate the effects of riluzole on obsessions, compulsions, and restricted interests/repetitive behaviors in this important patient population. Because this was the first controlled trial of riluzole in childhood-onset OCD, the NIH Combined Neurosciences Institutional Review Board (CNS-IRB) stipulated that only ‘treatment-resistant’ subjects could be enrolled. To be considered treatment resistant, the subject had to have failed or previously been unable to tolerate an adequate trial of at least one SRI. Further, it was required that if a child was prescribed any medical treatment for OCD, those medications should be continued, despite their failure to produce symptom remission. Thus, findings of this trial represent use of riluzole as an adjunctive therapy, rather than a typical placebo-controlled efficacy trial.
MATERIALS AND METHODS
This clinical trial was approved by the National Institutes of Health CNS-IRB. It was entirely supported by the Intramural Research Program of NIMH. Written informed consent and, where appropriate, assent were obtained for each subject in the trial. A Clinical Research Advocate from the NIMH Subjects Protection Unit monitored the consent process as well as research participation throughout the study.
Participants
Subjects were included in the study if they were between the age of 7 and 17 years, fulfilled the DSM-IV criteria for OCD, had a Children’s Yale-Brown Obsessive–Compulsive Scale (CY-BOCS; Scahill et al, 1997) score of ⩾20, and were treatment resistant. Subjects in the OCD and ASD group (OCD+ASD) fulfilled DSM-IV criteria for both OCD and an ASD (Autistic Disorder, Pervasive Developmental Disorder-Not Otherwise Specified, or Asperger’s Disorder). With one exception, children in this OCD+ASD group were verbal and thus able to provide history to support the diagnosis of OCD. In the case of the single functionally non-verbal exception, there was sufficient information from parent and school to support the diagnosis of OCD. Pregnancy was exclusionary, and contraception was required of all subjects who were sexually active. Other exclusionary criteria included psychotic disorders, eating disorders, medical instability, suicidality, and conditions contraindicating riluzole administration. Use of psychotropic medications and other psychiatric diagnoses, including depressive disorders, was permitted. Subjects were medically healthy based on physical examination and laboratory tests.
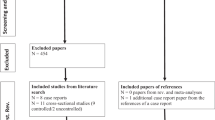
Seventy-two potential subjects were screened and twelve were excluded, so sixty subjects (mean age=14.47±2.35 years) were randomized to receive study drug (RIL) or placebo (PLA; Figure 1). The randomized sample was predominately male (n=44, 73%), had moderate-to-severe OCD symptoms (CY-BOCS ⩾20), and took concomitant medications (92%, mostly an SRI, Table 1). Seventeen subjects had OCD+ASD: autistic disorder, n=10; Pervasive Developmental Disorder Not Otherwise Specified, n=5; Asperger’s Disorder, n=2. No significant differences were observed between the study groups (RIL and PLA) on demographic or clinical characteristics at baseline; however, differences in scores between the study groups on the primary outcome measure, the CY-BOCS, approached significance (CY-BOCS Total: PLA, 29.13±3.59, RIL, 27.24±3.78; F(1,57)=3.9, p=0.053; CY-BOCS Obsessions: PLA, 14.57±1.98, RIL, 13.52±2.20; F(1,57)=3.7, p=0.06).
CONSORT diagram.
Design and Procedure
This was a 12-week, double-blind, placebo-controlled, randomized trial of adjunctive riluzole. Participants were outpatients; travel and lodging costs for each visit were paid by NIMH. Concomitant medications were required to be at stable doses for 2 months before randomization and throughout the controlled phase of the study. A planned blinded interim analysis conducted after 23 of the first 24 subjects completed the week 12 assessment (one RIL participant was lost to follow-up) indicated comparable changes in CY-BOCS scores between groups from baseline to week 2 ratings. Thus, IRB approval was obtained for modification of the study design to require a 2-week lapse between the screening assessment and the baseline (randomization) assessment with the purpose of excluding subjects who would be more prone to have a significant decrease in symptoms by randomization. The change was made to include only participants whose CY-BOCS scores were ⩾20 and no more than 20% improved from screening to baseline. Following this protocol change, none of the subsequently enrolled subjects failed to meet these randomization criteria.
The NIH Clinical Center Pharmacy prepared 10-mg capsules from 50-mg tablets purchased from the manufacturer, and also prepared look-alike placebo capsules. Study drug was initiated at one capsule (10 mg RIL or one PBO capsule; the minimum dose) daily, and was increased daily in a forced flexible dose design by one capsule until reaching the maximum dose of 100 mg/day (five 10-mg capsules, twice daily). The pharmacy used a table of random numbers to generate the randomization scheme. Subjects were stratified, using alternating blocks of 4 and 6, by diagnosis (OCD vs OCD+ASD). The subjects’ parents kept a diary of doses taken, time taken, time of meals, and adverse symptoms (if any). At each study visit (weeks 2, 4, 8, and 12), subjects had safety laboratory tests, including tests of liver, kidney, and pancreas function. Changes in other medications and any adverse effects also were documented. No CBT or structured psychotherapy was permitted during the trial.
Measures
Baseline study evaluation included physical examination, electrocardiogram, electroencephalogram, and laboratory studies. The diagnostic assessment included a comprehensive clinical interview with one or both parents (conducted by PG), and a semi-structured interview with parent(s) and subjects capable of participating (LL used The Schedule for Affective Disorders and Schizophrenia for School-Age-Children—Present and Lifetime version; Kaufman et al, 1997). In addition, the Autism Diagnostic Observation Schedule (Lord et al, 2000) and Autism Diagnostic Interview, Revised (Lord et al, 1994), administered by doctoral level staff trained to research standards, were used for the diagnosis and assessment of ASD symptoms. All participants (OCD and OCD+ASD) received the Autism Diagnostic Observation Schedule; if criteria for ASD were met on that measure, the Autism Diagnostic Interview, Revised was administered to parents.
The primary outcome measures in this study were the child- and parent-reported CY-BOCS, the Clinical Global Impressions-Severity and Improvement scales (CGI-S, CGI-I; Guy, 1976), and the Children’s Global Assessment Scale (CGAS; Shaffer et al, 1983). Interrater reliability (PG and LL) for the CGI scales and the CGAS was strong (intraclass correlation coefficients, 0.87–0.88).
Treatment response at week 12 was defined as at least 30% improvement from baseline on the total score of the CY-BOCS or reduction to subclinical levels of impairment (ie, a score of 2 (‘much improved’) or 1 (‘very much improved’) on the CGI-I scale).
Secondary outcomes were gathered at baseline and end point. The Repetitive Behavior Scale-Revised (Bodfish et al, 2000) is a 43-item caregiver report of repetitive behaviors. Self-report measures included the Multidimensional Anxiety Scale for Children (March et al, 1997) and the Child Depression Inventory (Helsel and Matson, 1984). In addition to laboratory safety measures, adverse events (AEs) were evaluated at every visit using a standardized clinician-administered checklist.
Statistical Analysis
Power analyses indicated that 26 subjects per study group were required to detect an effect size of d=0.80 with 80% power (two-tailed alpha set at 0.05). Data for all subjects with post-baseline assessment (RIL, n=29; PLA, n=30) were analyzed using the intent-to-treat (ITT) principle. Given the rate of discontinuation (RIL, n=8; PLA, n=1), parallel completer analyses were carried out on 51 subjects (85%). Primary outcome data from baseline and weeks 2, 4, 8, and 12, and secondary outcome data from baseline and week 12 were analyzed using a linear mixed model. Each model included fixed effects of study group, time, and the interaction between time and study group. Further, the baseline value of the outcome variable was included as a covariate in each of the primary outcome models. Given that baseline values were controlled for and interactions between time and study group were found to be non-significant in all analyses, the main effect of study group is reported. Restricted maximum likelihood estimation was used, and Schwarz’s Bayesian Criterion was used to select the autoregressive moving average model (1,1) as the best fitting structure. Secondary outcomes, assessed only at baseline and week 12, were analyzed using repeated-measures ANOVA on last-observation-carried-forward data. Response rate was compared between study groups using χ2. Between-group effect sizes for change at week 12 were calculated as Cohen’s d (Cohen, 1988).
Moderation, where the treatment effect differs between subgroups, is possible even when overall treatment effects are not observed. Thus, several variables were explored post-hoc as potential moderators of treatment effect. Each candidate moderator was a baseline variable, therefore independent from treatment assignment, and was entered as factorial expressions in the linear mixed model. A significant interaction between the candidate moderator and study group was the criterion for moderation. Candidate moderators were sex, age, baseline CY-BOCS severity, and the presence of ASD. Alpha (two-tailed) was set at p<0.05 for all analyses, which were completed using SPSS 19.0.
RESULTS
Fifty-one subjects (85%) completed the double-blind trial. One participant in the PLA group withdrew consent without providing an explanation. Eight participants (27%) of the RIL group discontinued participation; the reasons were as follows: lost to follow-up (n=1), elected to pursue treatment for pre-existing depression (n=1), pancreatitis (n=1), and elevated transaminase levels (protocol dictated discontinuation when these values were twofold higher than baseline values (n=2) or, following a protocol amendment, the upper limit of the reference range (n=3)). Data from 59 participants (RIL, n=29; PLA, n=30) were entered into the ITT model (Table 2). All participants were on 100 mg/day of riluzole/placebo at end point.
There was no effect of study group on change in the CY-BOCS total scores between baseline and week 12; average improvement in the RIL group (21±18%, 5.52±4.40 points) was very similar to that observed in PLA (19±15%; 5.83±4.86 points; F=0.04, p=0.84). No effect of study group was observed on any of the remaining primary outcome measures (Table 2). Finally, the proportion of responders did not differ between study groups using CGI-I (RIL=7%, PLA=7%; p=0.96) or CY-BOCS (RIL=16%, PLA=18%; p=0.69). No differences were observed between groups on the secondary outcome measures, gathered at baseline and end point of the double-blind trial (Repetitive Behavior Scale-Revised, Multidimensional Anxiety Scale for Children, and Child Depression Inventory; see Table 2). No significant interactions were observed between candidate moderator (ie, sex, age, baseline CY-BOCS severity, and the presence of ASD) and group, indicating that the treatment effects (or lack thereof) were not related to these variables (data available upon request). To explore the possibility that the ASD moderator analysis lacked power, the primary outcomes were assessed separately in OCD and OCD+ASD (data available upon request). Results were nearly identical in both groups, supporting the moderator finding that the presence of ASD had no bearing on response to riluzole.
Observed (not last observation carried forward) CY-BOCS total score data are illustrated in Figure 2. Results using only those who completed the study (n=51) were nearly identical to the ITT analyses; no differences were found between groups on the CY-BOCS, CGAS, and CGI-S.
Observed data and CY-BOCS total scores during double-blind study for RIL and PLA. Mean scores for CY-BOCS total score included 59 patients at baseline (RIL=29, PLA=30), 58 at week 2 (RIL=29, PLA=29), 58 at week 4 (RIL=28, PLA=30), 55 at week 8 (RIL=26, PLA=29), and 51 at week 12 (RIL=22, PLA=29).
A list of AEs by study group is available from the authors. There was no statistically significant difference between study groups in the occurrence of any AE. However, it is worth noting that one serious AE, pancreatitis, occurred at day 42 of RIL administration. The subject, who was taking three concomitant medications, had a complete and uneventful recovery. In addition, five subjects in the RIL group had asymptomatic elevations of transaminases at doses of 100 mg/day and were referred to a gastroenterologist; no sequelae were noted. Other laboratory tests as well as electrocardiograms did not show significant changes during the study.
DISCUSSION
Despite the variety of treatments available for childhood OCD, a sizable proportion of affected children remains impaired. Riluzole, a glutamatergic modulator, has preliminary data supporting its effectiveness in several adult psychiatric disorders (Zarate and Manji, 2008). Following a successful open-label trial (Grant et al, 2007), we hypothesized that riluzole would be effective for treatment of OCD in children. To our knowledge, this was the first randomized, placebo-controlled study of RIL in a pediatric population with OCD. Adjunctive riluzole was fairly well tolerated. Asymptomatic elevated transaminases led to discontinuation of riluzole in five subjects, but this was not associated with any sequelae and reversed rapidly to normal upon drug discontinuation (as previously described in adults, Zarate and Manji, 2008). For these subjects with treatment-resistant OCD, RIL was not found to be superior to placebo in reducing symptoms of OCD or global functioning, as measured by the primary (CY-BOCS, CGAS, and CGI) and secondary outcomes. In addition, there were no differences in response rates between groups. Post-hoc moderator analyses were undertaken, in order to explore the possibility that the treatment was more effective in a subgroup of our sample. No potential moderators, including sex, age, baseline CY-BOCS severity, and ASD diagnosis, were supported.
The result of this study demonstrated no significant differences between riluzole and placebo on any of the outcome measures. However, these results may not be generalizeable to all pediatric patients with OCD because of sample and design characteristics. First, enrollment was limited to treatment-resistant subjects with moderate-to-severe OCD symptoms. Indeed, the response rates in the present study were quite low for both CGI-I (RIL=7%, PLA=7%) and CY-BOCS (RIL=16%, PLA=18%). Depending on the response criteria, other studies in pediatric OCD have found response rates greater than 25% (Liebowitz et al, 2002; March et al, 1998; Riddle et al, 2001). One study found that 4% of the placebo group achieved a score of <10 on the CY-BOCS (Pediatric OCD Treatment Study, 2004), a criterion met by no members of PLA in the current study. Thus, it is possible that efficacy may have been noted with a less treatment-refractory group of patients.
Second, most participants (92%) were taking concomitant medications; nearly three-quarters took an SRI, and about half took an antipsychotic medication. Had riluzole been studied as monotherapy it may have separated from placebo. Third, the lack of efficacy with RIL may have been because the dose studied was too low. Riluzole has been tested in adult psychiatric disorders up to 200 mg/day; thus it is possible that further benefits in our children may have occurred with the use of higher doses—although most adults studied with RIL generally required doses between 100 and 150 mg/day. At end point, all children were on the maximum dose of 100 mg/day. Fourth, the difference at baseline between riluzole and placebo groups on the primary outcome measure (CY-BOCS total score) approached significance and may have contributed to the lack of separation between groups. However, this is an unlikely explanation as baseline values of all outcome measures were controlled for in the linear mixed models.
Finally, the length of treatment was consistent with other treatment studies of childhood OCD (Abramowitz et al, 2005; Watson and Rees, 2008). However, it may have been insufficient to observe an effect of riluzole. Clinical experience suggests that many patients with OCD begin to respond to SRI only at or around 12 weeks, so a longer study may have revealed an effect of riluzole treatment.
Psychotic disorders were exclusionary, but other diagnoses, such as depressive disorders, were allowed. This may have contributed to the placebo response. We elected to include a subset of participants with ASD in addition to moderate-to-severe OCD. Post-hoc moderator analyses showed that the 17 children with ASD were no more likely to respond to treatment of their OCD than were children without ASD. Importantly, riluzole had no specific benefits for the restricted and repetitive behaviors of ASD, suggesting that it would not be useful for the treatment of that core symptom.
Despite open-label data suggesting a therapeutic effect of riluzole on childhood OCD, this double-blind, placebo-controlled, 12-week study of adjunctive riluzole failed to demonstrate superiority of the drug over placebo. Several important characteristics of this study qualify the results, including the treatment-refractory nature of the sample as well as the presence of other psychiatric disorders (except psychotic disorders) and other pharmacologic treatments. Thus, future research might demonstrate an effect of riluzole monotherapy on moderate-to-severe symptoms of OCD in a less treatment-resistant sample. Although this study found no effect of treatment, it should not be considered the definitive study, and future investigations are needed to clarify the role of riluzole in childhood OCD.
FUNDING AND DISCLOSURE
Dr Zarate is listed as a co-inventor on a patent application for the use of ketamine and its metabolites in major depression (Intranasal administration of ketamine to treat depression. Application #: W02007US06898. The use of (2S,6S)-Hydroxynorketamine, (S)-Dehydronorketamine and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-Ketamine in the treatment of depression and neuropathic pain. E-092-2011/0-US-01; NIH0058US). Dr Zarate has assigned his rights in the patent to the US Government but will share a percentage of any royalties that may be received by the Government. The remaining authors declare no conflict of interest.
References
Abramowitz JS, Whiteside SR, Deacon BJ (2005). The effectiveness of treatment for pediatric obsessive-compulsive disorder: a meta-analysis. Behav Ther 36: 55–63.
Arnold PD, Sicard T, Burroughs E, Richter MA, Kennedy JL (2006). Glutamate transporter gene SLC1A1 associated with obsessive-compulsive disorder. Arch Gen Psychiatry 63: 769–776.
Bellingham MC (2011). A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade? CNS Neurosci Ther 17: 4–31.
Bodfish JW, Symons FJ, Parker DE, Lewis MH (2000). Varieties of repetitive behavior in autism: comparisons to mental retardation. J Autism Dev Disord 30: 237–243.
Cohen J (1988) Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum.
Dickel DE, Veenstra-VanderWeele J, Cox NJ, Wu X, Fischer DJ, Van Etten-Lee M et al (2006). Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Arch Gen Psychiatry 63: 778–785.
Farmer C, Thurm A, Grant P (2013). Pharmacotherapy for the core symptoms in autistic disorder: current status of the research. Drugs 73: 303–314.
Geller DA (2006). Obsessive-compulsive and spectrum disorders in children and adolescents. Psychiatr Clin North Am 29: 353–370.
Geller DA, Biederman J, Stewart SE, Mullin B, Martin A, Spencer T et al (2003). Which SSRI? A meta-analysis of pharmacotherapy trials in pediatric obsessive-compulsive disorder. Am J Psychiatry 160: 1919–1928.
Grant P, Lougee L, Hirschtritt M, Swedo SE (2007). An open-label trial of riluzole, a glutamate antagonist, in children with treatment-resistant obsessive-compulsive disorder. J Child Adolesc Psychopharmacol 17: 761–767.
Guy W (1976). ECDEU Assessment manual for psychopharmacology. In US Department of Health E, and Welfare (ed) National Institute of Mental Health: Rockville, MD.
Helsel WJ, Matson JL (1984). The assessment of depression in children: the internal structure of the Child Depression Inventory (CDI). Behav Res Ther 22: 289–298.
Javitt DC, Schoepp D, Kalivas PW, Volkow ND, Zarate C, Merchant K et al (2011). Translating glutamate: from pathophysiology to treatment. Sci Transl Med 3: 102mr102.
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P et al (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36: 980–988.
Liebowitz MR, Turner SM, Piacentini J, Beidel DC, Clarvit SR, Davies SO et al (2002). Fluoxetine in children and adolescents with OCD: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry 41: 1431–1438.
Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC et al (2000). The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30: 205–223.
Lord C, Rutter M, Le Couteur A (1994). Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24: 659–685.
MacMaster FP, O'Neill J, Rosenberg DR (2008). Brain imaging in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 47: 1262–1272.
March JS, Biederman J, Wolkow R, Safferman A, Mardekian J, Cook EH et al (1998). Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA 280: 1752–1756.
March JS, Parker JD, Sullivan K, Stallings P, Conners CK (1997). The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry 36: 554–565.
Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET (2008). Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev 32: 525–549.
Micali N, Heyman I, Perez M, Hilton K, Nakatani E, Turner C et al (2010). Long-term outcomes of obsessive-compulsive disorder: follow-up of 142 children and adolescents. Br J Psychiatry 197: 128–134.
Pediatric OCD Treatment Study (2004). Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA 292: 1969–1976.
Riddle MA, Reeve EA, Yaryura-Tobias JA, Yang HM, Claghorn JL, Gaffney G et al (2001). Fluvoxamine for children and adolescents with obsessive-compulsive disorder: a randomized, controlled, multicenter trial. J Am Acad Child Adolescent Psychiatry 40: 222–229.
Rosenberg DR, MacMaster FP, Keshavan MS, Fitzgerald KD, Stewart CM, Moore GJ (2000). Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. J Am Acad Child Adolesc Psychiatry 39: 1096–1103.
Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK et al (1997). Children's Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry 36: 844–852.
Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H et al (1983). A children's global assessment scale (CGAS). Arch Gen Psychiatry 40: 1228–1231.
Stewart SE, Geller DA, Jenike M, Pauls D, Shaw D, Mullin B et al (2004). Long-term outcome of pediatric obsessive-compulsive disorder: a meta-analysis and qualitative review of the literature. Acta Psychiatrica Scandinavica 110: 4–13.
Watson HJ, Rees CS (2008). Meta-analysis of randomized, controlled treatment trials for pediatric obsessive-compulsive disorder. J Child Psychol Psychiatry 49: 489–498.
Wink LK, Erickson CA, Stigler KA, McDougle CJ (2011). Riluzole in autistic disorder. J Child Adolesc Psychopharmacol 21: 375–379.
Wu K, Hanna GL, Rosenberg DR, Arnold PD (2012). The role of glutamate signaling in the pathogenesis and treatment of obsessive-compulsive disorder. Pharmacol Biochem Behav 100: 726–735.
Zarate CA, Manji HK (2008). Riluzole in psychiatry: a systematic review of the literature. Expert Opin Drug Metab Toxicol 4: 1223–1234.
Acknowledgements
This research (protocol 05-M-0225) was supported by the Intramural Program of the National Institute of Mental Health (NIMH) of the National Institutes of Health (NIH; Grant # 1ZIAMH002913). ClinicalTrials.gov identifier NCF00251303.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Grant, P., Joseph, L., Farmer, C. et al. 12-Week, Placebo-Controlled Trial of Add-on Riluzole in the Treatment of Childhood-Onset Obsessive–Compulsive Disorder. Neuropsychopharmacol 39, 1453–1459 (2014). https://doi.org/10.1038/npp.2013.343
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2013.343
Keywords
This article is cited by
-
A perspective on molecular signalling dysfunction, its clinical relevance and therapeutics in autism spectrum disorder
Experimental Brain Research (2022)
-
A Randomized Placebo-Controlled Cross-Over Pilot Study of Riluzole for Drug-Refractory Irritability in Autism Spectrum Disorder
Journal of Autism and Developmental Disorders (2018)
-
Aripiprazole and Riluzole treatment alters behavior and neurometabolites in young ADHD rats: a longitudinal 1H-NMR spectroscopy study at 11.7T
Translational Psychiatry (2017)
-
Evidence-Based Treatments in Treatment-Naïve and Treatment-Resistant Pediatric Obsessive-Compulsive Disorder
Current Behavioral Neuroscience Reports (2015)
-
Neue Studien zur Behandlung der Zwangsstörung
DNP - Der Neurologe und Psychiater (2015)