Abstract
The dopamine agonist pramipexole (PPX) can increase impulsiveness, and PPX therapy for neurological diseases (Parkinson's disease (PD) and restless leg syndrome) is associated with impulse control disorders (ICDs) in subpopulations of treated patients. A commonly reported ICD is pathological gambling of which risk taking is a prominent feature. Probability discounting is a measurable aspect of risk taking. We recently developed a probability discounting paradigm wherein intracranial self-stimulation (ICSS) serves as the positive reinforcer. Here we used this paradigm to determine the effects of PPX on discounting. We included assessments of a rodent model of PD, wherein 6-OHDA was injected into the dorsolateral striatum of both hemispheres, which produced persistent PD-like deficits in posture adjustment. Rats were trained to perform ICSS-mediated probability discounting, in which PD-like and control groups exhibited similar profiles. Rats were treated twice daily for 2 weeks with 2 mg/kg (±)PPX (ie, 1 mg/kg of the active form), a dose that improved lesion-induced motor deficits. In both groups, (±)PPX increased discounting; preference for the large reinforcer was enhanced 30–45% at the most uncertain probabilities. Tolerance did not develop with repeated treatments. Increased discounting subsided within 2 weeks of (±)PPX cessation, and re-exposure to (±)PPX reinstated heightened discounting. Such findings emulate the clinical scenario; therefore, ICSS for discounting assessments in rats exhibited high face validity. This model should prove useful in medication development where assessment of the propensity of a putative therapy to induce risk-taking behaviors is of interest.
Similar content being viewed by others
INTRODUCTION
Dopamine (DA) agonists pramipexole (PPX) and ropinirole are FDA approved for treatment of motor dysfunction in Parkinson's disease (PD) and restless leg syndrome (RLS). DA agonist therapy is associated with impulse control disorders (ICDs) in an estimated 14% of treated PD patients (Voon and Fox, 2007; Weintraub et al, 2010) and 7–12% of treated patients with RLS (Pourcher et al, 2010; Driver-Dunckley et al, 2007). These drugs are being used off label for other pathologies, including fibromyalgia and bipolar disorders wherein ICDs are also observed (Holman, 2009; Strejilevich et al, 2011). Independent of the pathology for which the therapy is implemented, ICD onset is reported to relate to onset of DA agonist treatment, and symptoms typically subside with dose reduction or discontinuation (Dodd et al, 2005; Driver-Dunckley et al, 2007; Mamikonyan et al, 2008; Quickfall and Suchowersky, 2007). In North America, ICDs associated with DA agonists commonly include problem/pathological gambling, compulsive sexual behavior, compulsive buying, and binge eating (Weintraub et al, 2010). These behavioral disorders are reward or incentive based and repetitive in nature (Evans et al, 2009), indicating that DA agonists can lead to dysregulation of general reward processes. Supporting this concept, acute PPX can enhance reward-mediated learning (Pizzagalli et al, 2008; Santesso et al, 2009) and impulsivity in healthy human volunteers (Riba et al, 2008; but see Hamidovic et al, 2008).
To better understand the link between DA agonists and ICDs, and to provide a means to screen new therapies without a propensity to induce aspects of impulsivity, a valid animal model is needed. Towards that end, we developed a novel probability discounting paradigm in laboratory rats (Rokosik and Napier, 2011). This task measures how changes in probabilities alter decision making. For example, subjects are given a choice between a small reward that is always delivered and a large reward that is sometimes delivered. If the probability of obtaining a large reinforcer is high, the subject will prefer the large reinforcer; however, lower probabilities will drive preference for the small reward. If discounting increases, this reflects a reduced importance of the low probabilities, and the subject will exhibit preference for the large reward during both high and low probabilities for reward obtainment. Thus, probability discounting is a popular method to study risky decision making, one facet of impulsivity. Problem gamblers demonstrate increased risk taking in probability discounting paradigms (Holt et al, 2003; Madden et al, 2009; Petry, 2011). To provide a potent, rapid, and reliable reward that allows for repeated tests of discounting, we employed intracranial self-stimulation (ICSS) as the positive reinforcer in rats (Rokosik and Napier, 2011). The ability for repeated testing is a critical feature for assessments of chronic treatments. As yet, laboratory evaluations have not yet been conducted for chronic PPX administration, and this is needed to better emulate the therapy scenario used clinically. To fill this gap, the current study evaluated the effects of chronic (±)PPX treatment on probability discounting. To emulate the pathology for which PPX is most often used clinically, we included assessments in a 6-hydroxydopamine-hydrobromide (6-OHDA) model of PD. As DA agonists, including PPX, are front-line therapy for early-stage PD (Bonuccelli et al, 2009), we sought to model the human brain at this stage, that is, when dopaminergic lesions are largely confined to the putamen (Kish et al, 1988). The rodent dorsolateral striatum (DLS) is the homolog of the primate putamen, and lesions of DA inputs to the DLS via 6-OHDA injections are a common way to model early stages of PD in rats (Deumens et al, 2002; Przedborski et al, 1995). Thus, we used this approach to provide a PD model in which to study the effects of ±PPX.
MATERIALS AND METHODS
Subjects
Male Sprague-Dawley rats weighing 250–274 g upon arrival (Harlan, Indianapolis, IN) were housed in pairs under environmentally controlled conditions (0700 h/1900 h light/dark cycle, temperature maintained at 23–25 °C) with access to rat chow and water ad libitum. Rats were handled according to federal standards. Protocols were approved by Rush University IACUC.
Treatment Drugs
Pramipexole (synthesized as the racemic mixture; Daya Drug Discoveries, Hazelwood, MO) (±PPX) was dissolved in saline and given intraperitoneally (IP) as 0.25, 0.5, 1.0, 2, or 4 mg/ml/kg for assessments in stepping and 2 mg/kg for the discounting task. To induce dopaminergic lesions, 6-OHDA (Sigma-Aldrich, St Louis, MO) was dissolved in 0.2% ascorbic acid in a sterile saline solution (pH=5.0) and infused into the striatum at a dose of 7.5 μg per 2 μl per side (as the salt). Rats were given a 30-min pretreatment of 25 mg/kg (as the salt) of desipramine-HCl (DMI; Sigma-Aldrich) dissolved in sterile water to reduced uptake of the 6-OHDA into adrenergic neurons.
Surgical Procedures for 6-OHDA Injections and Electrode Implantation
To stereotaxically lesion the striatum and implant the stimulation electrode, rats were anesthetized with sodium pentobarbital (50 mg/kg/ml IP; Sigma-Aldrich), administered DMI, and the head placed in a stereotaxic frame (David Koft, Tujunga, CA) with the nose piece set at 3.3 mm below the horizontal. A 33-gauge, bilateral injector was lowered to the DLS (1.0 mm anterior to bregma, 3.4 mm lateral from midline, 4.7 mm ventral from skull). At 30 min after DMI, 6-OHDA was injected at a rate of 0.2 μl/min for 10 min. Sham controls were similarly injected with the ascorbic acid vehicle. The injectors were left in place for an additional min (to allow the solution to diffuse away from the tip) and the skull holes were filled with bone wax. A bipolar stimulating electrode (MS303/3-B/SPC; Plastics One, Roanoak, VA) was lowered to the lateral hypothalamus (LH; 2.6 mm posterior to bregma; 1.8 mm lateral; 8.4 mm ventral). Electrodes were secured to the skull with stainless steel screws and dental acrylic, and the incision was sutured. Rats were allowed at least 5 days of recovery from surgery before operant testing was initiated.
Behavioral Testing
Motor assessment: forelimb adjusting step test
The 6-OHDA-induced motor deficits were verified using the forelimb adjusting step test, (Olsson et al, 1995) conducted 1 day before surgery and at least once a week after surgery. To do so, the experimenter suspended the rat's rear legs and one forelimb while the rat supported itself on its unrestrained forelimb. The rat was ‘dragged’ on the unrestrained forelimb 0.9 m for 5 s in abduction and adduction directions for both forelimbs, and the number of adjusting steps was counted. Three stepping trials were taken per session, and the average score was determined.
An initial study was conducted to validate the rat model of PD employed here with regard to (1) brain DA deficits, and (2) motor dysfunction for a time frame that would coincide with duration of the probability discounting paradigm. The 6-OHDA-treated rats were killed 21 days (n=6) or 60 days after lesion (n=6); sham rats (n=5) were killed 60 days after lesion. Forelimb stepping adjustments were measured every 3 days. Lesion extent was verified in ex vivo tissue harvested 21 or 60 days after 6-OHDA infusion using tyrosine hydroxylase immunohistochemistry (TH-IHC).
A separate group of lesioned rats (also implanted with stimulation electrodes) were used to conduct a (±)PPX dose vs stepping response evaluation. These rats were tested with the stepping task 1 day before surgery and every week after. At ∼40 days after the lesion, the following protocol was used: (±)PPX was administered to sham (n=7) and 6-OHDA-treated rats (n=5) in the morning and stepping adjustments were measured immediately before, and 1 and 6 h after treatment. In the evening, a second (±)PPX injection (of the same dose) was given and stepping was measured 17 h later. Treatments (vehicle, 0.25, 0.5, 1, 2, and 4 mg/kg, IP) were administered weekly in a pseudorandomized order.
ICSS Procedures and Apparatus
ICSS experiments were conducted in operant chambers (30.5 cm × 24.1 cm × 21.0 cm; Med-Associates, St Albans, VT) outfitted with a chamber light, and two retractable levers each under a stimulus light and enclosed in ventilated, sound-attenuated boxes. Electrical brain stimulation (EBS) was delivered by a programmable stimulator (PHM-152/2) via bipolar leads connected to commutators (Plastics One, Roanoak, VA) mounted above the chamber. Typically, two ICSS test sessions were conduced per day. The following describes the testing protocols for various phases in the probability discounting paradigm.
ICSS-Mediated Probability Discounting
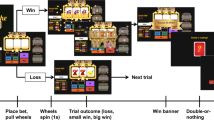
A nine-phase paradigm was used to determine rats' baseline discounting and effects of (±)PPX, as previously described (Rokosik and Napier, 2011). Table 1 shows the acquisition criteria for phases 1–6 that were required before initiating (±)PPX treatment (phases 7–9) in the current study. Briefly, the phases are described as follows. Phase 1, shaping. A single lever was extended and EBS (200 μs biphasic square wave pulses, applied at 100 Hz for 500 ms) was delivered. Only the initial current intensity (100 μA) was adjusted for each rat based on their performance to approach and ultimately press the lever. The final intensity level was used for the remaining phases. Phase 2, fixed ratio-1 (FR-1) reinforcement. To establish stable ICSS lever pressing, rats underwent a continuous FR-1 reinforcement schedule wherein one lever was extended for a 30-min session. Phase 3, rate-frequency function. Rats were pseudorandomly presented with 1 of 16 different current frequencies tested in 10 Hz increments, ranging from 10 to 160 Hz. Train duration and current intensity were held constant. For each frequency, rats had access to the lever for 2 min and the numbers of lever presses were recorded. Following each 2 min period, the lever retracted for 10 s. In each session, a lever pressing rate vs ICSS current frequency (termed the rate-frequency function) was collected and the maximal (Emax) and minimal (threshold) number of lever presses were determined using a nonlinear regression (GraphPad Prism, La Jolla, CA). When a rat met phase acquisition criteria (see Table 1), averages of three curves were used to determine ICSS frequencies that produced 90, 60, and 40% of Emax (termed effective current (ECur); ECur90, ECur60, and ECur40, respectively; see Figure 1). Phase 4, discrete trials. Rats were trained to recognize the temporal nature of trials using each rat's own ECur60 as the reinforcer. Each session comprised 200 trials. Trials occurred in 15 s intervals. Each session began with both levers retracted and the chamber light off; 2 s later, the chamber light was illuminated, followed 3 s later by the extension of one lever. The rat had 10 s to press the lever, if the response was not executed, the trial was aborted (termed an omitted trial), the lever retracted, and the chamber light turned off. If a lever press was made, an EBS was delivered and the stimulus light over the lever was turned on. After 0.5 s, all lights were turned off and the lever retracted. The two levers were alternately extended among trials. Phase 5, choice test. The purpose of this phase was to determine for each rat, a small and large reinforcer that could be used in the probability discounting phase. Using the FR-1 discrete trials described in phase 4, rats were trained to select from different, lever-specific, reinforcement values. Each session consisted of three blocks. Each block consisted of 20 forced-choice trials followed by 20 free-choice trials. In forced-choice trials, one lever was extended at a time allowing the rat to learn the reinforcement value associated with that lever. In free-choice trials, both levers were extended, and the rat had to choose between the lever-specific reinforcement values. Initially, small and large reinforcers corresponded to the rat's ECur90 and ECur40 (obtained in phase 3). To complete this phase, rats had to demonstrate a ‘free-choice ratio’ (the number of selections for the large reinforcer divided by the total number of lever responses made × 100) of at least an average of 70% across the three blocks. Phase 6, probability discounting task. Each session consisted of nine blocks as used in phase 5, but here, one lever was designated ‘small/certain lever’ (SC) and the other was ‘large/risky’ lever (LR). A press on the SC lever always delivered the small reinforcer (ie, approximately ECur40); a press on the LR lever delivered the large reinforcer (approximately ECur90) with varying probabilities. The following three series of probability presentations were cycled during this, and subsequent phases: (1) 0.5, 0.3, 0.85, 0.6, 0.05, 0.7, 1.0, 0.4, and 0.15; (2) 0.15, 0.6, 0.4, 0.05, 0.7, 0.3, 0.85, 1.0, and 0.5; and (3) 0.7, 0.4, 1.0, 0.15, 0.5, 0.85, 0.05, 0.3, and 0.6. For each series, the LR lever was designated either to the left or right lever; therefore, each rat experienced six different probability formats. Data from free-choice trials of each probability (ie, block) were analyzed to determine a baseline free-choice ratio vs probability function. If in a block, there were ⩾50% omissions from the free-choice trials (ie, >10 of 20 trials tested), data from that block were excluded from subsequent analysis. This criterion was held for phases 6–9 (each of which employed the probability discounting task), and overall, <2% of the blocks were excluded. Phase 7, (±)PPX treatment. At 1 day following the last baseline test, (±)PPX treatment was initiated. The regimen was 2 mg/kg (±)PPX, IP, twice a day (in the morning and evening) for 13 days (termed, chronic treatment). The ±PPX-induced changes in discounting were assessed 30 min and 6 h following the morning injection on the first and every third day of the chronic treatment. Phase 8, withdrawal. In a subset of rats, (±)PPX was withdrawn for 15 to 69 days after cessation of treatment. Phase 9, re-instatement. The (±)PPX treatment was reinitiated twice a day for 7 days. Probability discounting was assessed every third morning throughout phases 8 and 9.
ICSS rate-frequency function. In phase 3, the relationship between ICSS lever pressing rate and stimulation frequency (an index of signal strength) was obtained for each rat. Illustrated is the final curve (ie, met stability criteria as described in Table 1) for an individual PD-like rat. From this curve, the ECur90 (solid line), ECur60 (dotted line), and ECur40 (dashed line) were determined using a nonlinear regression (GraphPad Prism).
Histology and TH-IHC
Rats were deeply anesthetized with chloral hydrate (400 mg/kg; Sigma, St Louis, MO). A 5 V DC current was applied to the stimulating electrode for 30 s to deposit iron and/or produce a very discrete lesion at the electrode tip. The iron deposits were visualized by a blue coloration produced via trychloroacetic acid (0.5%) and potassium ferricyanide (3%) added to a 4% paraformaldehyde solution used for transcardial perfusion after perfusing with ice-cold 0.9% NaCl. Brains were removed, postfixed in 4% paraformaldehyde, and stored in a 30% sucrose solution. Brains were sliced in 40 μm coronal sections. Striatal sections were immunoreacted with a primary, monoclonal mouse anti-TH antibody (ImmunoStar, 22941) diluted 1 : 10 000 and a biotinylated horse anti-mouse IgG (Vector Laboratories, BA2001) diluted 1 : 100. The signal was amplified by avidin and biotinylated horseradish peroxidase using the Elite ABC Vectastain Kit (Vector Labs, PK6100). Immunostaining was visualized with 3,3-Diaminobenzidine tetrachloride dehydrate (Sigma, D5637) solution activated with 0.3% H2O2.
Data Analysis
To compare PD-like and control rats, data from phases 1 to 6 were analyzed using Student's t-test. A linear correlation was conducted between (1) lever pressing rate and EBS frequency (Hz; phase 3) to verify that changes in EBS frequency altered ICSS, and (2) free-choice ratio and probability magnitude (phase 6) to determine if the two groups acquired the discounting task. To determine treatment-induced changes in free-choice ratio collected in phases 6–9, a two-way repeated measures (rm) ANOVA was conducted.
For phases 6 and 7, day and probability were factors. For phases 8 and 9, phase and probability were factors. A post hoc Newman–Keuls provided individual comparisons. Forelimb stepping was similarly analyzed with time and dose as factors. If a data point exceeded two SD from the group mean, it was considered an outlier and it was excluded from analysis. Significance was p<0.05 for group/treatment comparisons; data are reported as group means±SEM.
RESULTS
Intradorsolateral Striatal Injections of 6-OHDA Produced Persistent Motor Deficits That Were Reversed by Pramipexole
We conducted an initial study to validate that the DLS infusions of 6-OHDA resulted in a lesion that was sufficiently robust and persistent to produce stable and enduring reduction of TH in the DLS, and in deficits in forelimb stepping, similar to a previous report (Chang et al, 1999). The DLS of 6-OHDA-treated rats showed profound reductions in TH staining that persisted for 60 days (Figure 2). For the six rats killed at 21 days after lesion, the tissue sections that showed the largest lesion extent were between +1.2 mm and +0.7 mm anterior to bregma, and the lesion could be detected from +2.2 mm to −0.26 mm. Although all rats had similar presurgery baseline stepping, those treated with 6-OHDA displayed stepping deficits in both left and right forelimbs when tested in both the adduction and abduction direction. These deficits, which were similar for 21 and 60 days after lesion, were ∼40–50% of that obtained from sham rats (see Table 2). These data were analyzed using a planned contrast two-way rmANOVA. For all four parameters tested, there was a significant (p<0.05) effect of treatment group and postsurgery time, and group by time interactions (Left abduction: group F2, 48=13.63, time F1, 48=193.95, interaction F2, 48=20.84; Right abduction: group F2, 48=14.21, time F1, 48=142.02, interaction F2, 48=15.78; Left adduction: group F2, 48=12.30, time F1, 48=146.04, interaction F2, 48=40.01; Right adduction: group F2, 48=3.30, time F1, 48=54.83, interaction F2, 48=18.37). This study verified that the 6-OHDA treatment protocol profoundly reduced dopaminergic innervation of the DLS and this lesion was sufficiently robust and enduring to produce stable deficits in motor function that persist for at least 60 days. Thus, this 6-OHDA treatment protocol was employed for the subsequent ICSS studies, and stepping adjustments of the left forepaw in the abduction direction were used as the representative motor index of the DLS lesion.
Dorsolateral striatal lesions. (a) Representative photomicrographs of tyrosine hydroxylase-immunohistochemistry (TH-IHC) at the level of the DLS (∼1.0 mm AP from bregma) in one hemisphere. Compared with sham (vehicle-injected; left), 6-OHDA reduced staining in the DLS at 21 days (middle) and 60 days (right) after treatment. Scale bar=1 mm. (b) Bilateral illustration of the extent and location of 6-OHDA-induced lesions 21 days after injection. For the six rats killed at this time, the tissue sections that were targeted during surgery (1.0 mm anterior to bregma) were analyzed by two observers. Each independently outlined the TH-like staining for the section for each rat. The outermost borders delineated by lack of staining were determined. Illustrated are the outlines for the largest lesion area from both observers (neuroanatomical plates modified from Paxinos and Watson, 1998). The borders of the lesion after 60 days were less discrete (see (a), far right); but in general, the lesion size was similar to that seen at 21 days.
To verify that the deficits remained throughout the 85 days needed to complete the study, a separate group of rats that completed the ICSS-mediated discounting paradigm (n=21) were also assessed for forelimb stepping each week after surgery. We determined that stepping remained at ∼17 steps/session for control rats and 4–5 steps/session for PD-like rats. Similar to the rats tested 60 days after lesion (discussed above), these motor deficits persisted throughout the study (ie, for 85 days, data not shown).
To evaluate the ability of (±)PPX to reverse 6-OHDA-induced motor deficits, rats that failed to meet acquisition phase criteria in the discounting paradigm (n=5/16 PD-like; 7/17 shams; refer to Table 1 for criteria) were used. For these rats, the presurgery baseline average of adjusting steps/session were 14–15 and this level was not altered in control rats by either vehicle treatment or any dose of (±)PPX tested (data not shown). In contrast, PD-like rats showed a significant effect of (±)PPX dose (F5, 20=34.17, p<0.01) and post-treatment time (F3, 60=316, p<0.01) and an interaction (F15, 60=46.88, p<0.01). As shown in Figure 3, at doses ranging from 0.5 to 4.0 mg/kg IP, (±)PPX improved stepping deficits in PD-like rats at 1 h after treatment; 1.0–4.0 mg/kg maintained stepping improvements for at least 6 h after treatment. Adjusting steps returned to pre-(±)PPX deficit levels 17 h after injection for all doses tested. The stepping deficit was not altered by vehicle or 0.25 mg/kg (±)PPX. The 2 mg/kg (±)PPX dose produced robust motor improvements that persisted for 6 h and yet was below maximal improvement seen. Furthermore, this dose was not sufficient to influence behavior at 17 h after injection (Figure 3). Therefore, the treatment given in the late afternoon was mostly cleared from the animals before the morning injection, and (±)PPX likely did not accumulate during the repeated injections. These outcomes guided the dosing protocol selected for the probability discounting paradigm, that is, 2 mg/kg (±)PPX (ie, 1 mg/kg of the active form), administered twice a day. This decision was also guided by reports that (1) 1 mg/kg of (−)PPX alters reward-mediated behavior, that is, enhances the reinforcing effects of cocaine (Caine et al, 1997) and (2) twice-daily injections of 1 mg/kg of (−)PPX in rats increases expression of forebrain D3 receptors (Maj et al, 2000), which are involved in ICDs and addictions (Heidbreder and Newman, 2010).
Motor deficits produced by intradorsolateral striatal injections of 6-OHDA are reversed by pramipexole. Illustrated is adjusting stepping from the left forelimb in the abduction direction for PD-like rats. At ∼40 days after the lesion surgery, rats underwent a series of weekly step tests. Pre-(±)PPX deficits (Before) were obtained immediately before the (±)PPX injection. The ±PPX reversed these stepping deficits in a dose-dependent manner. The (±)PPX significantly increased the number of adjusting steps with 0.5, 1, 2, and 4 mg/kg at 1 h, whereas at 6 h this increase was only seen with 1, 2, and 4 mg/kg. The number of adjusting steps returned to pretreatment levels 17 h after injection. No change from before injection was seen after an injection with vehicle or 0.25 mg/kg of (±)PPX. The post-hoc Newman–Keuls: *vs before (±)PPX injection. Arrows indicate times of (±)PPX injection.
PD-Like and Control Rats Performed Similarly in the Probability Discounting Paradigm
The post-mortem histological evaluations verified that rats completing the ICSS-mediated paradigm had electrode tip placements located in the lateral hypothalamus (Figure 4). To determine if PD-like rats differed from controls in any aspect of paradigm acquisition, performance in phases 1–6 was monitored and compared for the two groups (refer to Table 1). All rats quickly acquired stable ICSS lever pressing, and both groups lever pressed on an FR-1 at similar rates. Similarly, the ECur90, ECur60 and ECur40 obtained from each rat's ICSS rate vs current frequency curve did not differ between groups. The averaged rate-frequency functions for PD-like and control rats are graphically indicated in Figure 5. Both groups exhibited significant linear regressions (PD-like, r2=0.94, p<0.01; and control rats, r2=0.91, p<0.01). The two groups learned and met phase criteria for the discrete trials and the choice tests in a similar time frame (Table 1). All rats that entered phase 6 were able to learn the discounting task. Figure 6 illustrates that both groups acquired the probability discounting task in the first session of phase 6, as demonstrated by a reduction in selection of the LR lever as the probability for delivery of the large reinforcer decreased (PD-like rats: r2=0.73, p<0.01; control rats: r2=0.85, p<0.01). Although the range for individual rats to obtain stable baseline discounting was 3 to 6 days, as groups, both the PD-like and control rats met stability criteria in the first 3 test days. For these 3 days of discounting, control rats showed an effect of probability (F8, 216=47.89, p<0.01) but no day effect (F2, 27=0.32, p=0.73) or interaction (F16, 216=1.17, p=0.29). There were seven data points removed because of meeting statistical outlier criteria. Similarly, for PD-like rats, there was an effect of probability (F8, 240=62.6, p<0.01), without an effect of day (F2, 30=0.23, p=0.80) or interaction (F16, 240=1.3, p=0.20). There were eight data points removed because of meeting statistical outlier criteria. Thus, for both groups there was a direct relationship between reward probability and free-choice ratio that did not differ for the first 3 baseline test days. For this phase, 9 of 1134 total blocks had response omissions of ⩾50%, and were omitted from the free-choice ratio analyses.
Electrode placement for intracranial self-stimulation (ICSS). Illustration of the stimulation electrode tip location within the lateral hypothalamus (LH) for 6-OHDA-treated (open circles, n=11) and sham (closed squares, n=10) rats that completed the probability discounting paradigm. Neuroanatomical plates were modified from Paxinos and Watson (1998) and numbers indicate the distance in mm from bregma. Note that the LH regions stimulated were similar for both groups of rats.
ICSS rate-frequency functions: comparisons between 6-OHDA-treated and sham rats. The relationship between ICSS lever pressing rate and stimulation frequency was similar for 6-OHDA-treated (n=11) and sham (n=10) rats. Shown are the group means±SEM from stable curves generated by each rat. Plots are drawn as a third-order polynomial to visualize Emax and threshold.
Acquisition of the probability discounting task. During phase 6, 6-OHDA-treated (n=11) and sham (n=10) rats acquired the probability discounting task during the first training session. Illustrated are the group means±SEM for the percent selection of the large/risky (LR) lever (ie, free-choice ratio) vs the probability that the large reinforcer was delivered for the first discounting session. The plot is drawn as a linear regression.
Pramipexole Increased Discounting in the Probability Discounting Task
To determine if (±)PPX altered probability discounting, rats were treated with 2 mg/kg (±)PPX twice a day (approximately 0800 h and 1700 h) for 13 days during phase 7. Discounting was measured 30 min and 6 h after the morning injection approximately every 3 days. These data were compared with pretreatment baseline sessions that were similarly conducted twice a day. Thus, to control for the possible effects of time of day for testing on outcomes, morning baseline sessions were compared with the tests taken 30 min after (±)PPX (also a morning test), and evening baseline sessions were compared with the tests taken 6 h after the morning (±)PPX injection (refer to Figure 7). For PD-like rats, comparisons of free-choice ratio for morning baseline to 30 min after the 1st and 25th (±)PPX injection revealed enhanced discounting (Figure 7a). There was a significant effect of test (ie, baseline, 1st, and 25th injection of ±PPX; F2, 29=14.45, p<0.01) and probability (F8, 232=31.07, p<0.01) and an interaction (F16, 232=5.85, p<0.01). Similarly, comparison of evening baseline with 6 h following the 1st and 25th (±)PPX injection revealed that ±PPX-induced heightened discounting was sustained (Figure 7c), with a significant effect of test (F2, 30=28.78, p<0.01), probability (F8, 240=65.42, p<0.01), and the interaction (F16, 240=6.49, p<0.01). A post-hoc Newman–Keuls comparison revealed that discounting was most pronounced following the 25th (±)PPX treatment for both 30 min and 6 h after injection (Figure 7). Unexpectedly, some control rats exhibited a large number of trial omissions 30 min following the first treatment of (±)PPX. Observation of these rats in the operant boxes revealed that they were engaged in continuous stereotypic sniffing and licking of the floor metal bars, with some head bobbing. The behaviors abated 6 h after the (±)PPX injection. The rats became tolerant to the motor effects, for on the fourth day of treatment (and the second discounting test) they were fully engaged in the lever pressing task and discounting performance could be accurately evaluated. However, the acute motor confound precluded discounting assessments for the first, 30 min post-(±)PPX treatment in control rats. After the seventh injection (ie, the fourth day of (±)PPX treatment), control rats clearly demonstrated increased discounting, as the selection for the risky lever at the 0.05, 0.15, and 0.30 probabilities were greater than baseline by 31%, 25%, and 27%, respectively. Figure 7b illustrates the enhancement in discounting observed 30 min after the 25th injection for control rats. There was a significant effect of test (F1, 18=4.97, p=0.04) and probability (F8, 144=20.93, p<0.01) and an interaction (F8, 144=3.22, p<0.01). Comparison of evening baseline testing with the 1st and 25th injection for the 6 h period also showed a significant increase in risky behavior (Figure 7d), with a significant effect of test (F2, 26=22.34, p<0.01), probability (F8, 208=42.21, p<0.01), and an interaction (F16, 208=2.18, p<0.01). As illustrated in Figure 7d, a post-hoc Newman–Keuls comparison revealed that discounting was most pronounced following the 25th (±)PPX treatment. For this phase, 50 out of 2457 total blocks had response omissions of ⩾50%, and were omitted from the free-choice ratio analyses.
Pramipexole increased probability discounting. In phase 7, 6-OHDA-treated (n=11) and sham (n=10) rats received 2 mg/kg (±)PPX IP twice a day for 13 days for a total of 26 injections. Discounting sessions were conducted 30 min and 6 h after the morning (AM) injection, on the first and every third day after initiating the treatment. Data from these two sessions were compared with the pretreatment baseline (BL0) for the respective time periods. The (±)PPX increased discounting in 6-OHDA-treated rats tested after the first (±)PPX treatment and the 25th (±)PPX treatment at both (a) 30 min and (c) 6 h after injection. Similar increases in discounting were seen in sham rats (b) 30 min and (d) 6 h after (±)PPX treatment. Shown is the percent selection of the large/risky (LR) lever (ie, free-choice ratio) vs the probability that the large reinforcer was delivered. The post-hoc Newman–Keuls: *vs BL; #vs first injection.
To help interpret (±)PPX-induced changes in probability discounting, we evaluated the effects of the agonist on various behaviors that are critical for the discounting task. First, demonstrating that rats maintained their ability to discriminate among the reinforcement values (ie, no reward vs small reward vs large reward), we determined at various times during the (±)PPX treatment that responding in the Choice Test (ie, the phase 5 protocol) was preserved (ie, selection for the larger reinforcer was approximately ⩾70%) for both PD-like (n=9) and control (n=8) rats. Second, we determined the ability of (±)PPX to alter the reward values. Following the 13 days of (±)PPX in phase 7, a subset of rats (controls, n=5 and PD-like, n=3) continued to receive 2 mg/kg (±)PPX twice a day for 3 additional days and the lever pressing rate vs ICSS current frequency (ie, the phase 3 protocol) was assessed. The ECur90 was similar between baseline (as determined in phase 3) and chronic (±)PPX for both groups (controls paired t-test(4)=0.89, p=0.43; PD-like t-test(2)=2.5, p=0.13). However, (±)PPX increased the rate of lever pressing at the lowest ICSS frequencies with a decrease in apparent threshold for both groups (data not shown), and for the PD-like group there was an associated reduction in ECur40 (paired t-test(2)=7.35, p=0.02). This shift went from 100 Hz at baseline to 52 Hz after the 32nd (±)PPX treatment. Such a change was not seen in control rats (paired t-test(4)=1.2, p=0.3). As a collective, these evaluations indicated that even though the value of the small reward may have been enhanced by (±)PPX, the rats continued to recognize ECur40 as less than the ECur90 so as to correctly execute the Choice Test and linked Discounting Test throughout the chronic (±)PPX treatment protocol.
Discontinuation of Pramipexole Decreased Probability Discounting
Following discontinuation of (±)PPX treatment, rats were continually assessed for discounting in phase 8. No overt behavioral indices of withdrawal were observed (eg, body weight, grooming), and hereafter the term ‘withdrawal’ is used to indicate the absence of drug treatment, not a behavioral index. At 3 days following the last injection, both control and PD-like rats maintained an increase in preference for the LR lever; however, reductions in this LR lever preference were evident 15 days after treatment cessation. Within this time period, some rats began to show a decrease in general performance and an increase in omissions during the discounting task (ie, >10 omitted trials out the 20 total); therefore, these rats were removed from the study. Of the rats that maintained performance, eight were PD-like and three were controls. For the PD-like rats, after 15 days of (±)PPX withdrawal, selection for the LR lever decreased as compared with 30 min after the 25th (±)PPX injection (Figure 8a). There was a significant effect of phase (ie, withdrawal vs 25th (±)PPX injection; F1, 14=7.29, p=0.02), probability (F8, 112=16.96, p<0.01), and an interaction (F8, 112=2.24, p=0.03). Indeed, discounting during this withdrawal time was nearly indistinguishable from baseline behavior; at the three lowest probabilities (ie, 0.05, 0.15, and 0.3), rats respectively selected the LR lever 52%, 55%, and 59% of the time during baseline and 42%, 55%, and 65% during withdrawal from chronic (±)PPX. As illustrated in the inset of Figure 8a, the three control rats demonstrated similar reduction in discounting as observed in the PD-like rats. That is, after 15 days of withdrawal, control rats selected the LR lever 44%, 29%, and 43% of the time at the three lowest probabilities (ie, 0.05, 0.15, and 0.3, respectively), which was similar to baseline values of 39%, 50%, and 56%, respectively. During this (±)PPX withdrawal period, 4 out of 198 total blocks had response omissions of ⩾50%, and were omitted from the free-choice ratio analyses.
Withdrawal from pramipexole decreased probabilistic discounting whereas reinitiation of pramipexole reinstated the increase in discounting. Shown is the percent selection for the large/risky (LR) lever (ie, free-choice ratio) vs the probability that the large reinforcer was delivered. (a) Phase 8; (±)PPX treatments were terminated. Illustrated are data from 6-OHDA-treated rats (n=8). Discounting measured on days 12 and 15 of withdrawal were averaged for each rat, and group data were compared with discounting obtained 30 min after the 25th (±)PPX injection. Inset illustrates data from sham rats (n=3); smooth line indicates 25th injection of (±)PPX and dotted line indicates withdrawal phase. (b) Phase 9; ±PPX treatment was reinitiated in a subset of withdrawn 6-OHDA-treated rats (n=6). Rats received 2 mg/kg (±)PPX IP twice a day for 7 days for a total of 14 injections. Discounting measured on the last 2 withdrawal days was averaged for each rat, and group data were compared with discounting data collected after the 13th (±)PPX injection during reinitiation. The post-hoc Newman–Keuls: *vs withdrawal.
Reinitiation of Pramipexole Reinstated Increased Discounting
A subset of drug-withdrawn rats (n=6; all PD-like) maintained successful performance of the discounting task and thus were continually tested up to 69 days after treatment. Throughout this time period, discounting remained near baseline levels (Figure 8b). Subsequently, the twice-daily 2 mg/kg (±)PPX treatment was reinitiated. The increase in discounting was reinstated by the seventh day of treatment (ie, after the 13th injection; Figure 8b), with a significant effect of phase (ie, withdrawal vs reinstatement; F1, 10=6.38, p<0.03), probability (F8, 80=10.06, p<0.01), and an interaction (F8, 80=5.86, p<0.01). The increase in discounting seen with reinstatement of (±)PPX was very similar to that obtained during the initial (±)PPX treatment. Indeed, at the three lowest probabilities (ie, 0.05, 0.15, and 0.3) during the initial (±)PPX treatment, 30 min after the 25th injection, rats respectively selected the LR lever 77%, 72%, and 90% of the time, which is comparative with 79%, 90%, and 84% (respectively) taken 30 min after the 13th reinstatement injection. During the reinstatement assessments, 1 out of 54 total blocks had response omissions of ⩾50%, and these were omitted from the free-choice ratio analyses.
DISCUSSION
Probability discounting is a popular method to study risky decision making, and problem gamblers demonstrate increased discounting in these paradigms (Holt et al, 2003; Madden et al, 2009; Petry, 2011). The current study utilized our new rat model of probability discounting that employs ICSS as the positive reinforcer (Rokosik and Napier, 2011) to reveal that (±)PPX increased discounting. We also revealed that tolerance did not develop with repeated treatments, and responding was comparable between PD-like and control rats. Additionally, we verified that increases in discounting returned to baseline levels within 2 weeks of (±)PPX treatment cessation, and re-exposure to (±)PPX reinstated heightened discounting. These outcomes are in line with clinical reports wherein ICD onset is related to onset of DA agonist treatment, and symptoms typically subside with dose reduction or discontinuation (Dodd et al, 2005; Driver-Dunckley et al, 2007; Mamikonyan et al, 2008; Quickfall and Suchowersky, 2007). Thus, using ICSS for risk assessments in rats exhibits high face validity to the human experience with PPX.
ICSS provides an immediate and robust reward that does not suffer from satiety/tolerance, or cause any withdrawal-like symptoms. Using ICSS, as opposed to food reinforcement, proved to be exceptionally advantageous for evaluating the effects of chronic (±)PPX treatment on probability discounting. First, the ICSS-mediated discounting task was acquired by rats in the first test session, and stable baseline discounting was achieved in 3 days of testing. This contrasts food reinforcement discounting where typically 10 test sessions are needed for acquisition and 25–35 days are required to reach stable discounting behavior (St Onge et al, 2010; Ghods-Sharifi et al, 2009). Second, ICSS allows for testing several probabilities in a randomized order, a feature that is not successfully implemented with food-reinforced discounting (St Onge et al, 2010). Randomization encourages rats to continue selecting the LR lever even at very low probabilities (in contrast to what is obtained with protocols using predictable, descending probabilities; Rokosik and Napier, 2011). Thus, we were able to detect both increases and decreases in selection of the LR lever at the lowest probabilites, where the most robust discounting often occurs. Finally, in food reinforcement studies, animals typically are food deprived to motivate them to perform the operant tasks. Food restriction alters the behavioral effects of PPX (Collins et al, 2008), which could confound outcomes of discounting tests with the agonist. To summarize, ICSS afforded a means to unambiguously assess discounting during chronic drug administration, following subsequent, long-term cessation of treatment, and drug reinstatement, all in the same test subjects.
The current study demonstrated the ability of a rodent model of PD to perform a probability discounting task. Although PD-like rats were robustly and persistently impaired in the forelimb adjusting step test, they readily performed the lever-pressing tasks and they did not show any behavioral deficiencies in the acquisition or execution of the discounting paradigm. Moreover, the PD-like rats displayed similar profiles as controls with regard to the reinforcing properties of ICSS currents (as assessed in the lever pressing rate vs current frequency profiles) and basal discounting. These observations indicate that DA deafferentation of the DLS does not alter the capacity, or motivation, to perform ICSS-mediated probability discounting.
Acute PPX treatment in healthy humans can increase measures of impulsiveness (Riba et al, 2008) as well as disrupt reward-related learning (Pizzagalli et al, 2008; Santesso et al, 2009, but see also Hamidovic et al, 2008). As a therapeutic agent, PPX can promote problem gambling independent of the pathology for which the drug is prescribed (eg, PD (Seedat et al, 2000; Weintraub et al, 2010), RLS (Quickfall and Suchowersky, 2007; Tippmann-Peikert et al, 2007), fibromyalgia (Holman, 2009), and bipolar depression (Strejilevich et al, 2011)). It is unclear if these pathological conditions render individuals more susceptible to the impulsivity-related effects of PPX. Given that PPX is highly prescribed during the early stages of PD and reports suggest these patients have a relatively high incidence of PPX-induced ICDs (Weintraub et al, 2010), we included a model of PD in the current study. However, we demonstrated here that a brain state that models aspects of early stages of PD did not render rats more sensitive to the (±)PPX-induced effects. It should be noted that this lack of differentiation between PD-like rats and controls may reflect the relatively high dose of (±)PPX studied, and lower doses of the agonist may be able to discriminate the two groups. Our findings that (±)PPX increased discounting in control rats are in line with food-reinforcement studies using food-restricted intact laboratory rats, wherein (−)PPX increases preference for a gambling-like schedule of reinforcement (ie, variable ratio; Johnson et al, 2011). These converging preclinical findings support a link between PPX treatment and alterations in decision making with regard to discounting.
In humans tested in probabilistic choice tests, PPX can disrupt learning from negative outcomes (ie, when a reward is expected but not delivered; Cools et al, 2006; Bodi et al, 2009). In probability discounting, when the probability of delivery of the large reinforcer is very low (eg, 0.05, 0.15, and 0.3), the likelihood of not receiving a reward is at the highest. Negative outcomes during these low probabilities likely lessen the appeal of lever pressing for the large reinforcer and shift preference to the SC lever. This profile was seen in the current study for tests during baseline and withdrawal. In contrast, (±)PPX enhanced responding on the risky lever during low probability. This outcome is consistent with the agonist reducing the negative consequences of a non-rewarded response. A similar outcome might be predicted if (±)PPX reduced the value of ICSS reward; however, the ECur90 (current level used for the large reward of the rats) was not altered by chronic (±)PPX and the ECur40 (the small reward) was slightly elevated. Although we have recently determined with a condition place preference paradigm that (±)PPX can support reward-mediated associated learning (Riddle et al, 2010), outcomes from the current operant task suggest that (±)PPX may increase discounting by reducing the perceived negativity of unrewarded operant responses rather than enhancing the value of the reward associated with the risky lever. This interpretation is supported by clinical studies with functional magnetic resonance imaging (fMRI) that investigated the influence of PPX on reward prediction errors during a gambling task. A positive reward prediction error occurs if an unpredicted reward is encountered and negative reward prediction error occurs if a predicted reward is omitted. In one study, PD patients treated with PPX showed a correlation between increases in risk taking and impairments in the deactivation of the fMRI signal in the orbitofrontal cortex during trials with a negative prediction error (van Eimeren et al, 2009). This suggests that the subjects were impaired from learning in trials in which losing occurred. In another study, RLS patients treated chronically with DA agonists, including PPX, demonstrated increases in fMRI signaling in the ventral striatal during trials in which expected rewards were omitted (Abler et al, 2009). It is noteworthy that the PPX-induced effects were observed in all RLS patients tested, similar to the ability of (±)PPX to enhance discounting in all rats tested in the current study. Nevertheless, none of the RLS patients developed an ICD (Abler et al, 2009). This outcome underscores the fact that enhancement in discounting or risky behaviors is not equivalent to developing an ICD per se but likely represents a particular aspect of these complicated disorders.
Which receptors mediate the behavioral effects of PPX is unclear. PPX is a direct-acting DA agonist with a preference for the D3R subtype of DA receptors. For example, in in vivo rat studies using presumed D2R- and D3R-selective behavioral assays (ie, hypothermia and yawning, respectively), PPX is ∼30-fold selective for D3R over D2R (Collins et al, 2007), and 1.0 mg/kg (−)PPX is sufficient to activate both D2R and D3R (Collins et al, 2005, 2007, 2009). Thus, it is likely that both subtypes were engaged by 2 mg/kg dose of (±)PPX used in the current study. Indeed, both D2 and D3R have been implicated in reward-mediated behaviors (Heidbreder et al, 2005; Self, 1998) and impulsivity (St Onge and Floresco, 2009; van Gaalen et al, 2009; Buckholtz et al, 2010). Additional probability discounting studies including those with lower doses of PPX as well as receptor-subtype selective antagonists would aid in elucidating the particular receptor(s) involved in PPX-induced enhancement in discounting.
The (±)PPX shifted discounting in PD-like rats with a single injection; however, repeated treatments were required to reach maximal discounting. These findings indicate that acute occupation of relevant DA receptors is sufficient to enhance discounting; however, the adaptations in this system that were imposed with chronic administration may promote the effect. Chronic PPX treatments can lead to desensitization of DA neuronal D2/D3 autoreceptors (Chernoloz et al, 2009) and an increase in expression of D3R in dopaminoceptive regions (Maj et al, 2000). Whatever the mechanism, the neuroadaptations were reversible in the current study, for when (±)PPX treatment was discontinued for 2 weeks, discounting decreased near baseline levels. These findings concur with clinical reports showing that DA agonist-induced ICDs in humans can be eliminated with drug discontinuation (Macphee et al, 2009; Mamikonyan et al, 2008; Quickfall and Suchowersky, 2007; Dodd et al, 2005; Driver-Dunckley et al, 2007).
In summary, converging evidence suggests that PPX can influence the processing of rewards and drive decision making towards higher discounting and more risky choices. The animal model of (±)PPX-induced discounting presented here provides a valuable new means to elucidate the pharmacological and neurobiological underpinnings of this aspect of impulsivity. This model should prove useful in the development of novel therapeutics devoid of enhancing discounting as well as a means to screen current and future compounds for their potential to promote risky behaviors.
References
Abler B, Hahlbrock R, Unrath A, Gron G, Kassubek J (2009). At-risk for pathological gambling: imaging neural reward processing under chronic dopamine agonists. Brain 132: 2396–2402.
Bodi N, Keri S, Nagy H, Moustafa A, Myers CE, Daw N et al (2009). Reward-learning and the novelty-seeking personality: a between- and within-subjects study of the effects of dopamine agonists on young Parkinson's patients. Brain 132: 2385–2395.
Bonuccelli U, Del DP, Rascol O (2009). Role of dopamine receptor agonists in the treatment of early Parkinson's disease. Parkinsonism Relat Disord 15 (Suppl 4): S44–S53.
Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS et al (2010). Dopaminergic network differences in human impulsivity. Science 329: 532.
Caine SB, Koob GF, Parsons LH, Everitt BJ, Schwartz JC, Sokoloff P (1997). D3 receptor test in vitro predicts decreased cocaine self- administration in rats. Neuroreport 8: 2373–2377.
Chang JW, Wachtel SR, Young D, Kang UJ (1999). Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson's disease: studies on medial forebrain bundle and striatal lesions. Neuroscience 88: 617–628.
Chernoloz O, El MM, Blier P (2009). Sustained administration of pramipexole modifies the spontaneous firing of dopamine, norepinephrine, and serotonin neurons in the rat brain. Neuropsychopharmacology 34: 651–661.
Collins GT, Calinski DM, Newman AH, Grundt P, Woods JH (2008). Food restriction alters N′-propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine dihydrochloride (pramipexole)-induced yawning, hypothermia, and locomotor activity in rats: evidence for sensitization of dopamine D2 receptor-mediated effects. J Pharmacol Exp Ther 325: 691–697.
Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C et al (2007). Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology (Berl) 193: 159–170.
Collins GT, Truccone A, Haji-Abdi F, Newman AH, Grundt P, Rice KC et al (2009). Proerectile effects of dopamine D2-like agonists are mediated by the D3 receptor in rats and mice. J Pharmacol Exp Ther 329: 210–217.
Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J et al (2005). Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. J Pharmacol Exp Ther 314: 310–319.
Cools R, Altamirano L, D'Esposito M (2006). Reversal learning in Parkinson's disease depends on medication status and outcome valence. Neuropsychologia 44: 1663–1673.
Deumens R, Blokland A, Prickaerts J (2002). Modeling Parkinson's disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol 175: 303–317.
Dodd ML, Klos KJ, Bower JH, Geda YE, Josephs KA, Ahlskog JE (2005). Pathological gambling caused by drugs used to treat Parkinson disease. Arch Neurol 62: 1377–1381.
Driver-Dunckley ED, Noble BN, Hentz JG, Evidente VG, Caviness JN, Parish J et al (2007). Gambling and increased sexual desire with dopaminergic medications in restless legs syndrome. Clin Neuropharmacol 30: 249–255.
Evans AH, Strafella AP, Weintraub D, Stacy M (2009). Impulsive and compulsive behaviors in Parkinson's disease. Mov Disord 24: 1561–1570.
Ghods-Sharifi S, St Onge JR, Floresco SB (2009). Fundamental contribution by the basolateral amygdala to different forms of decision making. J Neurosci 29: 5251–5259.
Hamidovic A, Kang UJ, de WH (2008). Effects of low to moderate acute doses of pramipexole on impulsivity and cognition in healthy volunteers. J Clin Psychopharmacol 28: 45–51.
Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ et al (2005). The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Brain Res Rev 49: 77–105.
Heidbreder CA, Newman AH (2010). Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann NY Acad Sci 1187: 4–34.
Holman AJ (2009). Impulse control disorder behaviors associated with pramipexole used to treat fibromyalgia. J Gambl Stud 25: 425–431.
Holt DD, Green L, Myerson J (2003). Is discounting impulsive? Evidence from temporal and probability discounting in gambling and non-gambling college students. Behav Processes 64: 355–367.
Johnson PS, Madden GJ, Brewer AT, Pinkston JW, Fowler SC (2011). Effects of acute pramipexole on preference for gambling-like schedules of reinforcement in rats. Psychopharmacology (Berl) 231: 11–18.
Kish SJ, Shannak K, Hornykiewicz O (1988). Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N Engl J Med 318: 876–880.
Macphee GJ, Copeland C, Stewart D, Grosset K, Grosset DG (2009). Clinical follow up of pathological gambling in Parkinson's disease in the West Scotland study. Mov Disord 24: 2430–2431.
Madden GJ, Petry NM, Johnson PS (2009). Pathological gamblers discount probabilistic rewards less steeply than matched controls. Exp Clin Psychopharmacol 17: 283–290.
Maj J, Rogoi Z, Margas W, Kata M, Dziedzicka-Wasylewska M (2000). The effect of repeated treatment with pramipexole on the central dopamine D3 system. J Neural Transm 107: 1369–1379.
Mamikonyan E, Siderowf AD, Duda JE, Potenza MN, Horn S, Stern MB et al (2008). Long-term follow-up of impulse control disorders in Parkinson's disease. Mov Disord 23: 75–80.
Olsson M, Nikkhah G, Bentlage C, Bjorklund A (1995). Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci 15: 3863–3875.
Paxinos G, Watson C (1998). The Rat Brain in Stereotaxic Coordinates. Academic Press: New York.
Petry NM (2011). Discounting of probabilistic rewards is associated with gambling abstinence in treatment-seeking pathological gamblers. J Abnorm Psychol; e-pub ahead of print 15 August 2011.
Pizzagalli DA, Evins AE, Schetter EC, Frank MJ, Pajtas PE, Santesso DL et al (2008). Single dose of a dopamine agonist impairs reinforcement learning in humans: behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology (Berl) 196: 221–232.
Pourcher E, Remillard S, Cohen H (2010). Compulsive habits in restless legs syndrome patients under dopaminergic treatment. J Neurol Sci 290: 52–56.
Przedborski S, Levivier M, Jiang H, Ferreira M, Jackson-Lewis V, Donaldson D et al (1995). Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine. Neuroscience 67: 631–647.
Quickfall J, Suchowersky O (2007). Pathological gambling associated with dopamine agonist use in restless legs syndrome. Parkinsonism Relat Disord 13: 535–536.
Riba J, Kramer UM, Heldmann M, Richter S, Munte TF (2008). Dopamine agonist increases risk taking but blunts reward-related brain activity. PLoS ONE 3: e2479.
Riddle JL, Rokosik SL, Napier TC (2010). Pramipexole induces a conditioned place preference in parkinsonian-like and control rats. Program No 813 21, 2010. Neuroscience Meeting Planner, San Diego, CA: Society for Neuroscience, 2010 Online.
Rokosik SL, Napier TC (2011). Intracranial self-stimulation as a positive reinforcer to study impulsivity in a probability discounting paradigm. J Neurosci Methods 198: 260–269.
Santesso DL, Evins AE, Frank MJ, Schetter EC, Bogdan R, Pizzagalli DA (2009). Single dose of a dopamine agonist impairs reinforcement learning in humans: evidence from event-related potentials and computational modeling of striatal-cortical function. Hum Brain Mapp 30: 1963–1976.
Seedat S, Kesler S, Niehaus DJ, Stein DJ (2000). Pathological gambling behaviour: emergence secondary to treatment of Parkinson's disease with dopaminergic agents. Depress Anxiety 11: 185–186.
Self DW (1998). Neural substrates of drug craving and relapse in drug addiction. Ann Med 30: 379–389.
St Onge JR, Chiu YC, Floresco SB (2010). Differential effects of dopaminergic manipulations on risky choice. Psychopharmacology (Berl) 211: 209–221.
St Onge JR, Floresco SB (2009). Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology 34: 681–697.
Strejilevich SA, Martino DJ, Igoa A, Manes F (2011). Pathological gambling in a bipolar patient treated with pramipexole. J Neuropsychiatry Clin Neurosci 23: E2–E3.
Tippmann-Peikert M, Park JG, Boeve BF, Shepard JW, Silber MH (2007). Pathologic gambling in patients with restless legs syndrome treated with dopaminergic agonists. Neurology 68: 301–303.
van Eimeren T, Ballanger B, Pellecchia G, Miyasaki JM, Lang AE, Strafella AP (2009). Dopamine agonists diminish value sensitivity of the orbitofrontal cortex: a trigger for pathological gambling in Parkinson's disease? Neuropsychopharmacology 34: 2758–2766.
van Gaalen MM, Unger L, Jongen-Relo AL, Schoemaker H, Gross G (2009). Amphetamine decreases behavioral inhibition by stimulation of dopamine D2, but not D3, receptors. Behav Pharmacol 20: 484–491.
Voon V, Fox SH (2007). Medication-related impulse control and repetitive behaviors in Parkinson disease. Arch Neurol 64: 1089–1096.
Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V et al (2010). Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol 67: 589–595.
Acknowledgements
We thank Jennifer L Riddle for her excellent technical assistance. This work was supported by the Michael J Fox Foundation, the Parkinson's Disease Foundation, Parkinson's Research Center at Rush University, and the Daniel F and Ada L Rice Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Rokosik, S., Napier, T. Pramipexole-Induced Increased Probabilistic Discounting: Comparison Between a Rodent Model of Parkinson's Disease and Controls. Neuropsychopharmacol 37, 1397–1408 (2012). https://doi.org/10.1038/npp.2011.325
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2011.325
Keywords
This article is cited by
-
Noradrenergic contributions to cue-driven risk-taking and impulsivity
Psychopharmacology (2021)
-
Motor impulsivity and delay intolerance are elicited in a dose-dependent manner with a dopaminergic agonist in parkinsonian rats
Psychopharmacology (2020)
-
Increased motor impulsivity in a rat gambling task during chronic ropinirole treatment: potentiation by win-paired audiovisual cues
Psychopharmacology (2019)
-
The neurobiology of impulse control disorders in Parkinson’s disease: from neurotransmitters to neural networks
Cell and Tissue Research (2018)
-
Impulse control disorders in Parkinson’s disease
Journal of Neural Transmission (2018)