Abstract
In this review, we consider affective cognition, responses to emotional stimuli occurring in the context of cognitive evaluation. In particular, we discuss emotion categorization, biasing of memory and attention, as well as social/moral emotion. We discuss limited neuropsychological evidence suggesting that affective cognition depends critically on the amygdala, ventromedial frontal cortex, and the connections between them. We then consider neuroimaging studies of affective cognition in healthy volunteers, which have led to the development of more sophisticated neural models of these processes. Disturbances of affective cognition are a core and specific feature of mood disorders, and we discuss the evidence supporting this claim, both from behavioral and neuroimaging perspectives. Serotonin is considered to be a key neurotransmitter involved in depression, and there is a considerable body of research exploring whether serotonin may mediate disturbances of affective cognition. The final section presents an overview of this literature and considers implications for understanding the pathophysiology of mood disorder as well as developing and evaluating new treatment strategies.
Similar content being viewed by others
INTRODUCTION
The Rise of Affective Neuroscience
Affective processing is fundamental to human behavior. All our actions and decisions occur in an emotional context, and therefore cognitive functions are colored by emotional state. Conversely, cognitive factors critically modulate affective responses; an obvious example being the effects of learning on social and moral emotions. In his influential book, Descartes' Error, Antonio Damasio (1994) argued that feeling is more fundamental than logical thought in understanding human behavior. In the ensuing 15 years, there has been a proliferation of studies exploring affective processing and interactions between affect and cognition, facilitated by the advent of brain imaging techniques that allow us to access emotional function directly. This is particularly crucial for basic emotional responses without direct behavioral measures. However, functional neuroimaging has revolutionized the study of all aspects of affective function, allowing us to measure brain responses to emotional information, and thus to develop brain-based models of affective cognition (Dolan, 2002).
Affective Cognition
There is a rich literature in experimental animals exploring emotional function. Paradigms eliciting aversion, fear, and stress are at the heart of this approach and have provided valuable and sophisticated information about the neurobiology and neurochemistry of emotion. Many of these paradigms have been adapted for use in human studies. However, we will not review this area herein; rather we focus on the interface between cognition and emotion, a concept that we consider to encompass processing of emotionally salient information in contexts requiring cognitive evaluation to generate an appropriate response. Thus, we will discuss tasks in which emotional stimuli must be categorized, remembered, attended, or actively inhibited, but not tasks designed primarily to elicit emotional responses (eg, anticipatory anxiety paradigms). Some of these paradigms, particularly those with highly evocative stimuli, may also elicit changes in the emotional state; however, we will not focus on this aspect. We will use a neuropsychological organizing principle based on distinct tasks targeting different underlying processes to undertake a data-driven review of the burgeoning literature on cognitive processing of affective information. We will include studies of social and moral emotion, as well as categorization and affective bias tasks. Emotional decision making, often dependent on rewards and punishments, is a related area but will be explored elsewhere in this volume and therefore we will not review it herein.
Affective Function and Mood Disorder
Disturbance of affective cognition constitutes a core aspect of the symptomatology of many psychiatric disorders, in particular depression and anxiety. Although these disorders are characterized by various cognitive and emotional disturbances, impairments at the cognitive/affective interface may be most specific to mood disorder (Clark et al, 2009) and may represent the most plausible targets for psychological and pharmacological therapies. Arguments that affective cognition represents a central efficacy marker for antidepressant treatment are gathering momentum (Harmer et al, 2010) and represent a major theme in current biological psychiatry research. We will focus primarily on unipolar depression, although frequent comorbidity with anxiety (Gorman, 1996) and commonalities of symptomatology and treatment response indicate a strong case for considering the disorders together in a neurobiological context (Ressler and Mayberg, 2007). Bipolar depression is a distinct disorder largely beyond our scope (see Phillips et al, 2008 for an excellent recent review).
Summary
In this review, we will consider the neurobiology of affective cognition, encompassing emotion recognition, categorization and bias, and social and moral emotion processing, which brings together these subcomponents in an ecologically valid context. Classic neuropsychological evidence will be discussed, pointing to core roles of the amygdala and ventral prefrontal cortex (PFC) in affective cognition. We will then consider the functional neuroimaging literature in healthy subjects, which elaborates the roles of these, and other, structures in affective cognition. There is a wealth of data in this area and our review, although inevitably selective, attempts to adopt a data-driven rather than a theoretically driven approach. The main aim of this article is to consider impairments of affective cognition in mood disorders, measured behaviorally and with neuroimaging. Finally, we consider the role of serotonin (5-hydroxytryptamine, 5-HT) in modulating affective cognition, which has important implications for understanding the pathophysiology of depression and mechanisms of effective antidepressants.
MEASURING HUMAN AFFECTIVE COGNITION
Fundamental Processes
Affective cognition, operationally defined as reflecting an interface at which emotional and cognitive processes are integrated to generate behavior, includes a number of important subprocesses. Thus, the perception and recognition of emotional valence is vital for many tasks. In some contexts, it is also important to label or categorize distinct emotions. There is also an important distinction between tasks in which emotions are explicitly the focus of cognition (eg, emotion identification) and tasks in which emotional content is irrelevant to behavioral response, or is designed to distract from cognitive processing. In the latter situation, optimal performance requires suppression of emotional response and the behavioral measure typically reflects how successful this suppression has been. In this section of the review, we will briefly introduce key paradigms used in the study of human affective cognition which incorporate these fundamental processes to different extents. Broadly, these can be divided into tasks using emotive images (or sounds), face processing tasks, and affective bias tasks. We also discuss social and moral emotion tasks which bring together all the subcomponents of affective cognition in a subtle nuanced context that reflects the true complexity of real-world affective cognition.
Processing of Emotional Images
Emotion perception tasks have been widely used with neuroimaging and typically involve viewing (or listening to) emotional material. For example, the International Affective Pictures Series (IAPS: Lang et al, 2008) are a set of pictures that have been extensively standardized using dimensional measures of valence and arousal and used to assess emotion perception in neuroimaging contexts. Some paradigms require subjects to make explicit judgments about the valence and/or intensity of the emotional content, thus tapping into emotion recognition, and to some extent categorization processes. Other paradigms require a nonemotional judgment, eg, asking subjects whether scenes are indoors or outdoors. In this review, emotional information is irrelevant to the decision, and a reaction time measure provides an index of whether subjects successfully suppress task-irrelevant emotional material. Paradigms with highly evocative stimuli (images, music, or film clips are all used) can also induce short-term changes in mood state and this represents an important confound to address.
Face Processing Tasks
Ekman and Friesen (1971) proposed that six basic emotional expressions could be recognized across both species and cultural boundaries: happy, sad, fearful, angry, disgusted, and surprised; and they developed a standardized set of stimuli on the basis of these emotions. Tasks using these stimuli, or variants, have emerged as among the most widely used probes of affective cognition. Typically, these stimuli do not elicit discernible changes in the emotional state. The stimuli can be used explicitly to assess emotion labeling or categorization, asking subjects to determine which emotion is expressed. For example, a morphed face paradigm uses computer-generated mixtures of a basic emotion with neutral expression such that the emotional content varies from 0 to 100% in increments of 10% (Young et al, 1997). Subjects are asked to identify emotions at different levels of intensity, providing an index of sensitivity. Again, these stimuli can also be used in covert paradigms in which subjects are asked to perform a task that does not depend on emotion recognition (such as gender identification), in which it is likely that suppression mechanisms are tapped.
Memory and Attention Bias Tasks
There is an extensive literature studying the effects of emotional valence of stimuli on ‘cold’ cognitive processes, particularly memory and attention. Memory bias tasks may study recognition or recall, explicit or implicit memory, as well as memory for faces, pictures, words, or other stimuli; however, the core feature is that stimuli have emotional valence. Typically, tests compare memory for positive, negative, and neutral stimuli, although some studies use only positive or negative valence. One example is the classic task of Cahill and McGaugh (1995) involving two stories with almost identical structures, but very different emotional content. Recall for the two versions of the story provides an index of emotional modulation of memory.
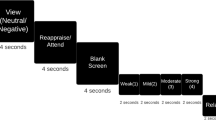
Attentional bias paradigms are usually adaptations of classic attention tasks to include an emotional component. Typical examples are emotional Stroop paradigms (in which subjects must name the color in which words, some of them emotional, are written), dot-probe paradigms (in which subjects must detect a target dot as quickly as possible, with emotional and neutral stimuli used to bias attention toward or away from the subsequent dot location), and affective Go/No-Go tasks. Different affective Go/No-Go tasks can involve facilitation of response to emotional targets, suppression of response to irrelevant emotional distracters, or (most typically) a combination of the two (see Figure 1).
Tasks used to assess attentional bias. This figure shows an emotional Stroop (panel a), affective Go/No-Go (panel b), and dot-probe task (panel c). Bias is indicated by facilitation of reaction time by emotional valence (as indicated).
Social and Moral Emotions
The tasks described above tap into the core components of affective cognition in different ways and may probe important mechanisms underlying mood disorders. However, it is important to recognize that real-world affective cognition is typically more complex than anything we assess in the experimental setting. Affective cognition is at the heart of successful social function, which is compromised in psychiatric disorder. To function socially depends on emotion recognition, categorization, attention, suppression, and memory, and therefore more complex social and moral emotion paradigms that tap into these processes are crucial models of real-world affective cognition. Moral emotions are cognitively multifaceted (Eisenberg, 2000) and entail complex causal attributions (Weiner, 1985), such as who caused an outcome. Sometimes they are referred to as ‘social’ emotions, because they depend on social interactions. However, one could argue that basic emotions are also ‘social’ emotions, and we therefore prefer the term ‘moral’ emotions to stress the fact that, in contrast to basic emotions, they motivate morally appropriate behavior. Moral sentiments enable people to overcome self-interest and benefit other people directly (eg, giving money to a beggar) or to follow or enforce moral rules and values that benefit society as a whole (eg, engaging in paper recycling). There is evidence for transcultural ubiquity of moral emotions (Fessler, 1999), but the exact actions and values for which we feel a certain moral emotion such as guilt greatly varies across sociocultural groups and individuals (eg, engaging in paper recycling is only just emerging as a widespread moral rule across the world).
Individual differences in proneness to certain moral emotions, such as guilt (O'Connor et al, 1997; Tangney, 1990) and shame (Tangney, 1990) have been classically measured using self-report questionnaires. Moral emotions can also be experimentally induced either in response to verbal descriptions or pictures of specific interpersonal behavior (Moll et al, 2007) or by verbal descriptions of acting counter to or in accordance with abstract social values (Zahn et al, 2009b). Having induced feelings by using such pictures or sentences repeatedly, participants are asked to pick a label among several alternatives that best describes their feeling (Moll et al, 2007; Zahn et al, 2009b). More recently, shame proneness has been measured implicitly (Rusch et al, 2007) circumventing social desirability biases classically encountered in self-report measures.
NEUROPSYCHOLOGICAL STUDIES OF AFFECTIVE COGNITION
Neuropsychological studies have been a fundamental component of developing models of nonaffective cognition. Our understanding of memory and language, eg, are infinitely richer for the many neuropsychological studies with amnesic and aphasic patients, and highly sophisticated models and theories have been derived from this research. However, neuropsychology has provided far less information about emotional processing and affective cognition, largely because of the rarity of lesions with selective effects on affective processing. Although rare, two specific types of lesion have been associated with disruptions of affective cognition: ventromedial prefrontal cortex (VMPFC) and amygdala lesions.
Ventral Frontal Lesions and Affective Cognition
The seminal neuropsychological case from an affective processing standpoint is Phineas Gage who suffered VMPFC damage in 1848 and was reported to show emotional and social changes in the absence of significant cognitive impairment (Damasio et al, 1994). More modern patients with orbitofrontal cortex (OFC) lesions have also been described (Damasio et al, 1994; Stuss et al, 1986; Cicerone and Tanenbaum, 1997), and social impairments dependent on emotional functions are commonly reported. Experimental studies of ventromedial patients have identified core deficits in reward- and punishment-guided decision making (Bechara et al, 1994; Rogers et al, 1999; Clark et al, 2008; Moretti et al, 2009), hypothesized as reflecting impairments in social and emotional decision making (Clark et al, 2008). However, there have been few explicit studies of affective cognition in ventromedial patients using paradigms discussed in this review, other than in the social and moral domains. Mah et al (2004) reported impairments in using social cues to make interpersonal judgments. Shamay-Tsoory and Aharon-Peretz (2007) assessed the performance of ventromedial patients on a social task, using stories with varying emotional content. They found that patients were specifically impaired on understanding stories as affective content increased. Shamay-Tsoory et al (2009) suggest that more cognitive aspects of empathy are differentially sensitive to ventromedial lesions, whereas emotional aspects depend on the inferior frontal gyrus.
Moral emotions have also been shown to be particularly sensitive to ventral frontal lesions; changes in moral attitudes, character, and behavior have been classically observed (Eslinger and Damasio, 1985; Welt, 1888). Ventral frontal lesions have been shown to decrease the ability to feel embarrassment (Beer et al, 2006) and regret (Camille et al, 2004). Patients with ventromedial frontal lesions (including the frontopolar cortex) decide less ‘emotionally’ and more ‘rationally’ in hypothetical moral dilemmas. For example, they would decide to push an innocent man to death on the tracks of a runaway trolley to save five other individuals more often than would controls (Ciaramelli et al, 2007; Koenigs et al, 2007). One explanation for this finding is that emotional moral decisions depend on the VMPFC, and when this is damaged, the cognitive dorsal frontal system can more easily enforce rational moral decisions that lead to overall greater moral benefit (Greene et al, 2004). Another explanation, supported by recent fMRI evidence (Kedia et al, 2008; Moll et al, 2007; Takahashi et al, 2004; Zahn et al, 2009a, 2009b), is that VMPFC is necessary for the expression of sympathy and guilt more than for other critical feelings, such as indignation or moral anger.
Moral emotion deficits are not unique to patients with ventromedial lesions. Patients with lesions to mesolimbic and basal forebrain regions may also exhibit morally inappropriate behavior (Moll et al, 2005). Neurodegeneration of the anterior temporal and ventral frontal lobes are common causes of morally inappropriate behavior and lack of emotional concern (Bozeat et al, 2000; Edwards-Lee et al, 1997; Mendez et al, 2005; Neary et al, 1998). Neurodegeneration of the right anterior temporal lobes was independently associated with morally inappropriate behavior and lack of empathy (Liu et al, 2004; Rankin et al, 2006).
Amygdala Lesions and Affective Cognition
Focal damage to the amygdala in humans is extremely rare, although amygdalectomy has historically been used in the treatment of both intractable epilepsy and extreme aggression (Narabayashi et al, 1963). Patients with amygdala damage can recognize faces and generate a normal range of facial expressions (Anderson and Phelps, 2000). However, they have deficits in tests of facial emotion recognition (Adolphs et al, 1994, 1999). Patients who were shown fearful faces generated significantly lower ratings of the fear expressed than did controls. This finding generalized to other negative emotions to some extent, but not to happiness (Adolphs and Tranel, 2004). Patients also showed impairments in interpreting expressions of social emotions such as guilt (Adolphs et al, 2002) and in complex social judgments based on facial expression (eg, how trustworthy the person was) (Adolphs et al, 1998). These findings suggest that the amygdala has a critical role in face emotion processing, perhaps by controlling response in posterior perceptual regions (Vuilleumier et al, 2004). Gosselin et al (2007) reported a case study of a patient with amygdala damage who was impaired at recognizing scary and (to a lesser extent) sad music, suggesting that the amygdala's role in emotion recognition extends beyond evaluating face information, or even information from the visual domain. A recent study strikes a further note of caution in interpreting these findings in terms of a fundamental role for the amygdala. Philippi et al (2009) used sophisticated imaging techniques to identify damage to white matter association tracts in brain-injured patients and reported that these tracts are critical to emotion recognition, even in the absence of focal amygdala damage.
Studies of patients with amygdala lesions have also shown that emotional memory is compromised. Hamann et al (1997) showed that a group of amnesic patients with varying lesion types presented mild-to-moderate memory deficits on measures of verbal recall, but still showed enhanced recall of emotional versions of the Cahill story task. By contrast, memory for the emotional story was selectively disrupted in patients with bilateral amygdala damage and spared hippocampus (Adolphs et al, 1997; Markowitsch et al, 1994). Boucsein et al (2001) reported that the extent of amygdala damage related to degree of impairment in an emotional associative learning task. Further evidence suggests that specifically left lateralized damage abolishes the normal enhancement of verbal memory by emotional valence (Buchanan et al, 2001; Edith Frank and Tomaz, 2003) whereas the right amygdala may mediate a similar effect for visual information (Markowitsch, 1998).
Summary
Neuropsychological studies indicate that the amygdala has a central role in emotion recognition and categorization, as well as emotional biasing of memory. Deficits in these processes may also contribute to higher-order social emotional deficits, such as impaired empathy and theory of mind (Stone et al, 2003). Patients with VMPFC damage do not appear to show deficits in basic affective cognition (although there is a paucity of empirical data), rather their impairments are in the social emotional domain. Although our understanding of affective cognition derived from neuropsychology lacks the sophistication of models in cognitive domains such as memory, evidence suggests that the amygdala, VMPFC, and the connections between them are crucial. Functional neuroimaging provides a tool to test and develop this hypothesis.
NEUROIMAGING OF NORMAL AFFECTIVE COGNITION
Emotional Images and Music
Emotional pictures and film clips have been used to study emotion perception from the early days of functional imaging. Lane et al (1997) reported that both pleasant and unpleasant IAPS pictures were associated with enhanced blood flow in regions, including the medial PFC and thalamus. Pleasant emotion additionally activated a part of the caudate. Unpleasant emotion additionally activated the parahippocampal gyrus, hippocampus, and amygdala. Film clips elicited similar patterns of response (Paradiso et al, 1997).
Recent studies have explored responses to emotional pictures in greater detail, to develop a more sophisticated understanding of the neuronal basis of emotion perception (see Phan et al, 2002; Wager et al, 2003; Sergerie et al, 2008 for reviews). Many studies focus on the role of the amygdala in perception of both negative (Wang et al, 2005; Stark et al, 2007) and positive (Garavan et al, 2001; Kensinger and Schacter, 2006) emotions. Regions specific to positive and negative valence have also been identified, in particular roles for the ventrolateral prefrontal cortex (VLPFC) (Kensinger and Schacter, 2006) and insula (Anders et al, 2004; Lewis et al, 2007) in response to negatively valenced pictures and words. Viinikainen et al (2010) explicitly explored correlations between subjective valence ratings and brain responses to positive and negative pictures (an explicit recognition and labeling task). Response in a number of regions correlated positively with valence ratings of unpleasant pictures, and response in partially overlapping regions correlated negatively with ratings of pleasant pictures, suggesting that the relationship between rating valence and BOLD response is a complex one. Furthermore, in a debate largely beyond our scope, it has recently been suggested that valence and arousal interact in critical emotion-processing regions (Lewis et al, 2007; Nielen et al, 2009).
Happy and sad music provide auditory analogs to positive and negative pictures, respectively, and fMRI studies also implicate emotion-processing regions in subjective response to emotive music (Brattico and Jacobsen, 2009). Koelsch et al (2006) reported that unpleasant (dissonant) music evoked response in the amygdala, hippocampus, parahippocampal gyrus, and temporal poles. By contrast, pleasant music evoked responses in regions, including the inferior frontal gyrus and ventral striatum. Similarly, Green et al (2008) reported that sad melodies were associated with increased activity in the left parahippocampal gyrus, bilateral ventral ACC, and left medial PFC. As all good film directors know, combining music and visual information can provide a more powerful emotional stimulus than either in isolation. Congruent emotional music enhances response to affective pictures in the amygdala, hippocampus, insula, striatum, and ventral frontal cortex (Baumgartner et al, 2006). Indeed emotional music combined with affectively neutral pictures is associated with enhanced response in these regions compared with music alone (Eldar et al, 2007).
Studies using evocative images or music are likely to confound emotion recognition/labeling and subjective experience of emotion. For example, Mitterschiffthaler et al (2007 reported fMRI responses to happy and sad music that were specifically associated with transiently induced happy and sad mood, respectively. Therefore, understanding the neuronal basis of emotion recognition in these rich contexts involves disambiguating recognition from subjective experience. Reiman et al (1997) used either film clips or personal recall to generate emotional response and found that internal and external generation of emotion depended on subtly different brain regions. A more direct attempt to separate perception of emotional content from subjective experience of emotion found that amygdala response was specific to perception, whereas hippocampal and prefrontal responses were associated with subjective experience (Garrett and Maddock, 2006).
Face Processing Tasks
Imaging studies of affective cognition have relied heavily on measuring responses to emotional faces, often using the Ekman stimuli. In general, emotional faces elicit enhanced response in the limbic, frontal, and visual cortical regions compared with neutral faces (Haxby et al, 2002; Ishai et al, 2004; Vuilleumier et al, 2001, 2004; Winston et al, 2003). One major avenue of enquiry has been the extent to which neuronal responses to different emotions can be distinguished. The literature is inconsistent, and at times contradictory, and in this review, we highlight some findings of interest rather than trying to give a comprehensive review. Amygdala response to fearful faces has been widely reported (Whalen et al, 2001), with increasing intensity of fearful expression resulting in increasing activity (Morris et al, 1996). Fearful faces also elicit response in the thalamus, ACC, and anterior insula (Morris et al, 1998). Angry faces elicit responses in the temporal cortex and PFC, including the ACC (Blair et al, 1999; Sprengelmeyer et al, 1998). Sadness, by contrast, elicits right-sided temporal lobe and amygdala responses (Blair et al, 1999). Disgust is associated with response in areas of the basal ganglia (Posamentier and Abdi, 2003) and reliable response in the insula, correlating with increasing intensity of disgust (Phillips et al, 1997). Increasing intensity of happiness has been associated with decreasing amygdala response (Morris et al, 1996). Of the six basic emotions, surprise is the least investigated and has distinct qualities. In particular, surprise can be described as a ‘transitory emotion’; it is briefly experienced and changes into other expressions depending on the nature of the surprise (Posamentier and Abdi, 2003). Kim et al (2003) investigated brain responses to surprise in relation to valence ratings; negative ratings of surprised faces elicited the right amygdala response, whereas positive ratings were associated with signal changes in the ventral PFC.
Recent studies have explored neuronal responses to emotional faces in more subtle ways, particularly in the context of fearful faces wherein amygdala response tends to be most reliably observed. Studies have focused on how interconnections between regions may critically subserve face emotion processing (Vuilleumier and Pourtois, 2007), using connectivity modeling techniques (Fairhall and Ishai, 2007; Ge et al, 2009; Goulden et al, 2010). For example, Fairhall and Ishai (2007) reported that coupling between the fusiform gyrus and the amygdala during face recognition was enhanced by fearful emotion. Meanwhile, Stein et al (2007) reported that responses to fearful and angry faces were associated with reciprocal connections between the amygdala and the ventral cingulate and also connections between the amygdala and the OFC.
There are also several studies exploring which aspects of face emotion are critical in determining neuronal response. In particular, studies of fearful face processing have focused on the importance of the eyes in conveying fear information, and amygdala response to fearful faces has been related specifically to directing gaze toward the eyes (Gamer and Buchel, 2009). Finally, there is increased evidence that exact cognitive and attentional demands of a task may be critical determinants of response to emotional faces. The pattern of response to faces depends on whether tasks require overt or covert processing of emotion (Phillips et al, 2004). Concurrent cognitive activity also has a critical role. Pessoa et al (2005) varied the difficulty of a central attentional task to explore how responses evoked by emotional faces depended on this manipulation. Amygdala responses to emotion were stronger during a gender identification task (when faces were attended) than during a bar-orientation task (when faces were unattended). Similarly, Silvert et al (2007) showed that emotional processing of unattended peripheral faces in the amygdala depended on the attentional demands on the main task, ie, the more demanding the task, the less the amygdala response. Brassen et al (2010) also reported suppression of amygdala response to faces as attentional resources were diverted elsewhere, and in this study, the effect was specific to fearful faces. These studies emphasize the importance of the interaction between emotional and cognitive factors and suggest a possible explanation for discrepancies in the literature, as they indicate that different face tasks will differentially engage emotional regions. In particular, the studies suggest that overt categorization of emotion is likely to engage emotional-processing regions differently from covert tasks. Thus, responses to emotional faces can only be understood in the context of specific cognitive demands.
Affective Bias (Memory and Attention)
A number of studies have introduced a post hoc memory component to emotion perception and recognition tasks. Thus, Canli et al (2000) reported that amygdala response to emotionally intense negative scenes was predictive of subsequent recall. Mickley Steinmetz and Kensinger (2009) showed that subsequent memory for negative or high arousal information was associated with occipital and temporal activity, whereas memory for positive or low arousal information was associated with frontal activity. Emotional biasing of memory can also be studied more directly using versions of classic memory bias tasks. Retrieval memory for emotional compared with neutral pictures is associated with enhanced response in the anterior temporal cortex and amygdala (Dolan et al, 2000). In a more subtle design, Smith et al (2004) showed that the neural basis of memory for neutral pictures was modulated by the emotional context in which they had been encoded, an incidental task in which emotional content is not the focus of cognition. Again, the amygdala and OFC were critical regions. Kuchinke et al (2006) examined PFC responses to word recognition. Emotional words were better remembered, associated with differential responses in subregions of the PFC. The right VLPFC correlated with correct retrieval of negative words, whereas the right VMPFC and OFC showed enhanced responses to positive words. Recall of faces is also modulated by emotion (Sergerie et al, 2007; Satterthwaite et al, 2009). Connectivity techniques have been used to examine mechanisms of affective modulation of memory in more detail. Smith et al (2006) showed that retrieval of emotional information depended on enhanced connectivity from the hippocampus to the amygdala. When retrieval of emotional material was relevant to current behavior, enhanced connectivity between the amygdala and the hippocampus was bidirectional and modulated by OFC. This study shows the importance of contextual relevance of emotional modulation of memory.
Emotional biasing of attention has also been studied using fMRI. Whalen et al (1998) reported preferential response of the ventral ACC to emotionally toned words in an emotional Stroop paradigm. More recent studies have reported enhanced ACC response specifically to positive words, and further, have related enhanced ACC response to the personality trait of extraversion (Canli et al, 2004; Haas et al, 2006). Etkin et al (2006) also highlighted a role of the rostral ACC in emotional Stroop performance, suggesting that the critical function of this region is in resolving emotional conflict. Emotional Stroop tasks require subjects to inhibit responses to emotional valence, and therefore these studies explore the neuronal basis of incidental processing of emotional valence, or a relative failure to inhibit emotionally salient information. Using an affective Go/No-Go task, Elliott et al (2000) also identified the ventral ACC as a critical area for modulating emotional biasing effects on cognition, a finding corroborated by a recent study (Chiu et al, 2008). Another study of healthy adults that used a Go/No-Go task with affective facial expressions as cues found slowed responses to fearful expressions that were associated with amygdala activation and difficulty in inhibiting responses to happy faces that was inversely related to caudate nucleus activity (Hare et al, 2005). These affective Go/No-Go studies require subjects both to attend and to categorize emotional content to identify targets and also to inhibit emotional response to nontargets. In a more complex face Go/No-Go design, considering interactions between emotional context and stimulus valence, Schulz et al (2009 reported valence-dependent response in the amygdala, insula, and posterior midcingulate, and an interaction between inhibition and emotion in the right inferior frontal gyrus and left posterior insula.
Social and Moral Emotion
As discussed above, social and moral emotion tasks bring together the subprocesses tapped by the tasks above, involving recognition and categorization of emotion, attention to relevant attentional cues, inhibition of irrelevant emotional cues, and memory of previous emotional contexts. Early fMRI investigations of moral emotion have contrasted activation in response to passive viewing of morally relevant vs morally irrelevant pictorial (Moll et al, 2002) or verbal materials (Moll et al, 2002). A neural network of the anterior ventral frontal/frontopolar cortex, the anterior temporal lobe, and the posterior superior temporal sulcus was selective for morally relevant materials. Activations were observed in this network irrespective of task demands: explicit moral evaluation (deciding whether morally right or wrong; Moll et al, 2002) or passive viewing (Berthoz et al, 2002; Moll et al, 2002). Frontopolar foci of activation were similar across studies (Greene et al, 2001; Heekeren et al, 2003; Moll et al, 2002). Another approach has been to investigate specific moral emotions, such as guilt or indignation. In this study, we focus on functional imaging studies of guilt which are particularly important for understanding melancholic depression and wherein some consistency across studies is emerging. The first neuroimaging study of guilt revealed ACC activation dorsally to the genu of the corpus callosum (Shin et al, 2000). Subsequent studies have shown frontopolar activation most consistently and when comparing against different control conditions (Kedia et al, 2008; Moll et al, 2007; Takahashi et al, 2004; Zahn et al, 2009b), including equally unpleasant feelings while blaming others: indignation/anger toward others).
When modeling the effect of individual differences in guilt proneness (Zahn et al, 2009b) or empathic concern (Zahn et al, 2009a), activation within the subgenual cingulate emerged as selective for guilt compared with indignation. As this region suffers from signal drop out in fMRI studies, it may be underreported when not using specifically tailored MRI protocols. In addition, the subgenual cingulate and the adjacent septal area were found to selectively respond when participants choose to donate to charities for which they feel compassion compared with a condition in which they gain money for themselves (Moll et al, 2006). Subsequent studies have also found activations within the septal area including the septal part of the nucleus accumbens for donation behavior (Harbaugh et al, 2007; Hsu et al, 2008) and for trust in economic interactions (Krueger et al, 2007). One hypothesis is that the subgenual cingulate and basal forebrain areas, such as the septal region, host-specific representations of affiliative punishment and/or rewards, and associated contexts which are hypothesized to be an integral part of feelings of guilt, sympathy, and altruistic motivations in general (Moll and Schulkin, 2009).
Summary: Neural Models of Normal Affective Cognition
We have briefly reviewed neuroimaging studies of affective cognition in healthy subjects. This review is far from exhaustive but emphasizes certain key points. First, it is clear that although the amygdala is a central structure in emotional processing (Costafreda et al, 2008), the ACC and OFC are also critical substrates of the affective-cognitive interface. Second, it is evident that the exact demands of the tasks (whether emotion should be attended or ignored, degree of cognitive control required, etc.) are important factors in determining the extent to which these, and other, structures are engaged. Third, more recent studies emphasize the importance of connectivity approaches to understanding critical interactions between emotion and cognition (Pessoa, 2008). Thus, it is not just region-specific responses of the amygdala, ACC, or OFC that are important, but rather the strength of connectivity between these regions, and their modulatory control of other regions. For example, modulation of amygdala–hippocampal connections seems to be a key substrate of emotional memory (Strange and Dolan, 2006), whereas connectivity between the amygdala and the visual cortex is critical in emotion face perception (Vuilleumier and Pourtois, 2007) (see Figure 2).
Key connections of the amygdala that may be differentially probed by affective cognition paradigms. It must be noted that this figure is a schematic only and does not purport to display all amygdala connections or the interconnections between any other components.
Distributed neural connectivity underpinning affective cognition has formed the basis for recent neural models of emotion processing. Ochsner and Gross (2005, 2007) propose a model of the cognitive control of emotion that focuses on interacting bottom-up and top-down systems. The bottom-up system is responsible for emotional appraisal and centers on the amygdala and striatum. Meanwhile, the top-down system exerts cognitive or regulatory control and depends on prefrontal structures. The authors distinguish two types of top-down controls: the dorsal PFC providing description-based appraisal and reappraisal and the ventral PFC providing outcome-based appraisal critical for learning associations between choices and consequences. Phillips et al (2003) proposed a similar model focusing on two systems. They hypothesized a ventral system involved in identifying emotionally salient stimuli, mediating autonomic responses and generating an emotional state. This system is mediated by the amygdala, insula, striatum, and ventral PFC (including the ventral ACC and OFC). Meanwhile, a dorsal system, including the hippocampus and dorsal PFC, is involved in cognitive control processes, such as selective attention and performance monitoring, as well as voluntary regulation of emotional states. Phillips et al (2008) have subsequently refined their model and have proposed that a lateral prefrontal system (DLPFC and VLPFC) may subserve voluntary aspects of emotional regulation, whereas a medial system (OFC, ACC, DMPFC) may subserve more automatic aspects. They proposed that both systems are active in normal regulation of emotions generated by emotion perception processes dependent on subcortical systems (such as the amygdala, striatum, and thalamus). Models of the interaction between emotion and cognition provide a basis for exploring disturbances associated with mood disorders. Although these models have proved valuable heuristics, they are typically based on conceptually driven reviews of selected aspects of the affective cognition literature. For example, Phillips et al (2003) explicitly proposed a model of emotion perception rather than affective cognition more generally. We adopted a data-driven approach in this review, and this broader review highlights important inconsistencies and limitations in the literature to date. In particular, apparently subtle variations in task demands can have significant effects on neuronal mechanisms. We suggest that it is premature to propose a comprehensive neuronal model of affective cognition on this basis.
AFFECTIVE DISTURBANCE IN MOOD DISORDER
Introduction
The clinical picture
Anxiety and low mood are part of normal experience, typically in response to future threats and recent losses, respectively (Finlay-Jones and Brown, 1981). Common forms of clinical depression and anxiety that evoke help seeking and treatment can be seen as excessive reactions to aversive experiences that prevent normal functioning. Indeed, recent psychosocial adversity (life events) often has a triggering role in timing the onset of depression, but mainly in the context of earlier psychosocial or familial vulnerability (Brown et al, 1987, 1995; Farmer and McGuffin, 2003). Anxiety disorders and depression commonly coexist (Kessler et al, 2003) and anxiety symptoms often precede the onset of depressive symptoms and outlast them (Wittchen et al, 2003). Twin studies suggest a substantial genetic overlap between depression and generalized anxiety disorder (GAD) (Kendler et al, 1992), although other evidence suggests they are nevertheless distinct disorders (Beesdo et al, 2010).
The core and required feature for a diagnosis of depression is low mood or sadness. However, loss of interest and inability to experience everyday pleasures (anhedonia), which can be more salient than sadness, is regarded as an equivalent to depressed mood. Anxiety symptoms include worry, apprehension, tension, and irritability (Fava et al, 2010). Disturbances in homeostatic (‘neuro-vegetative’) functions include insomnia, loss of appetite, dangerously reduced fluid intake in severe cases, and loss of libido. Psychomotor inefficiency includes slowing of movement and thought, as well as loss of concentration. The content of depressive ruminations and preoccupations is neatly summarized by Beck's cognitive triad; the self is worthless, life is pointless, the future hopeless (Beck, 1967). However, depressive cognition is not invariable; depression can be severe but experienced as undeserved suffering.
Vulnerability
Genetic predisposition accounts for 30–50% of the risk of depression and social factors such early life adversity (abuse, parental neglect) are important and interacting elements of vulnerability (Hill et al, 2001). They influence brain development and personality in many ways that in turn increase the risk of depression. There is good evidence that cognitive and emotional personality styles antedate the onset of depression. Neuroticism is a personality trait of emotional lability with a strong genetic component, which greatly amplifies the risk of depression with increasing levels of life stress (Kendler et al, 2004). Neuroticism is proving to be a useful personality dimension in understanding genetic and neurocognitive mechanisms of vulnerability to depression in drug-free nondepressed individuals (Chan et al, 2008, 2009). We recently reported that variation in the CNR1 cannabis receptor gene explained significant variance in neuroticism and disagreeableness scores and increased the risk of depression after life events (Juhasz et al, 2009). It is clear that vulnerability involves many genetic and psychosocial factors each potentially contributing to various mood, anxiety, and personality disorders but if present together at critical periods, they combine to produce cognitive and neurochemical vulnerability to the state of depression.
Distinct cognitive and emotional styles may also outlast recovery to predict risk of relapse. For example, the Dysfunctional Attitudes Scale (DAS) assesses negative attitudes based on Beck's triad. High scores in remitted asymptomatic patients predict greater risk of recurrence and are associated with neurochemical changes (see below; Thase et al, 1992). The tendency to ruminate and brood is another cognitive risk trait for relapse (Nolen-Hoeksema, 2000). Cognitive therapy targets such abnormal thinking styles both in the treatment of depression and in the prevention of relapse.
Disturbances of Affective Cognition
Abnormalities of face processing
Deficits in identifying facial emotions have been consistently reported in depression (Feinberg et al, 1986; Rubinow and Post, 1992; Persad and Polivy, 1993). Some studies have argued for a general decrease in sensitivity to emotional faces (Leppanen, 2006; Rubinow and Post, 1992; Mikhailova et al, 1996; Csukly et al, 2009; Hale et al, 1998). More specifically, others have argued that depressed patients exhibit negative biases in identifying facial emotions; patients are more likely to interpret neutral faces as sad and happy faces as neutral, resulting in an overall negative bias compared with controls (Gur et al, 1992; Surguladze et al, 2004). Using face emotion stimuli of varying intensity, Gilboa-Schechtman et al (2002) found that depression was associated with greater sensitivity to sad faces and greater response bias toward labeling faces as sad. By contrast, depressed patients had reduced sensitivity toward happy faces, needing more intense expressions to correctly label happiness (Joormann and Gotlib, 2006; Harmer et al, 2009). Coupland et al (2004) replicated this finding and showed that it correlated positively with anhedonic depression. Biased response to emotional faces has also been linked to the course of depression. The level of negative emotion perceived in schematic faces in a current depressive episode correlated with depression levels 3 and 6 months later (Hale, 1998) and predicted subsequent relapse (Bouhuys et al, 1999). Thus, depressed patients seem to show decreased sensitivity to emotional faces, but within that context, a negative bias. The effect observed experimentally may depend on task parameters.
Disrupted affective biases (attention and memory)
Depression has long been associated with a pattern of cognition biased toward negative information, with depressed individuals showing a tendency to remember negative items and to rate experiences or stimuli as more negative than do controls (Gur et al, 1992; Mogg et al, 1995). Negative biases have been hypothesized to have a role in the etiology and maintenance of depression (Beck et al, 1979) and seem to be more specific to depression than many of the nonemotional cognitive deficits observed. Presented with lists of emotionally toned words, many studies have shown that depressed or dysphoric subjects preferentially remember negatively toned material (Dunbar and Lishman, 1984; Ruiz-Caballero and Bermudez, 1995; Bradley et al, 1995, 1996; Rinck and Becker, 2005; Direnfeld and Roberts, 2006). Other studies have suggested that the negative bias observed in depression manifests itself as the absence of normal positive bias ( Ellwart et al, 2003; Gilboa-Schechtman et al, 2002; Harmer et al, 2009). Still further studies have failed to demonstrate significant biases (Danion et al, 1995; Bazin et al, 1996; Banos et al, 2001). One reason for these discrepancies is the nature of the tasks used. Barry et al (2004) argues that conceptually, rather than perceptually, driven tasks are more likely to elicit mood-congruent biases. Thus, biases are more likely when subjects perform a higher level of semantic processing (eg, word association compared with word-stem completion). Inconsistencies may also reflect subject characteristics, with ‘patient’ groups ranging from dysphoric students to severely depressed inpatients.
In addition to negatively biased memory in laboratory paradigms, overgeneral autobiographical memory has been repeatedly demonstrated in depression since it was first described by Williams and Broadbent (1986). Depressed patients were significantly slower than controls to produce personal memories in response to positive cues, with no group differences in response latencies to negative cues. Lemogne et al (2006) reported a similar autobiographical memory impairment specific to positive events. However, in a meta-analysis of 14 studies of overgeneral autobiographical memory, van Vreeswijk and de Wilde (2004) found that depressed mood seemed to moderate the overgenerality of both positive and negative memory.
Although it was traditionally believed that negative biases in depression were seen for memory rather than attentional tasks, clear evidence has emerged suggesting that attention is also biased toward negative information (Gotlib et al, 2004a, 2004b). For example, depressed patients show elevated interference (reflected by slower reaction times) in response to negatively valenced words in an emotional Stroop task (see Gotlib and McCann, 1984; Segal et al, 1995; Broomfield et al, 2007; Williams et al, 1996 for a review). Using a different attentional paradigm (affective Go/No-Go), Murphy et al (1999) found that depressed patients were slowed compared with controls when responding to happy but not to sad targets, which represents a bias away from positive information. A similar pattern was subsequently observed in never-medicated patients (Erickson et al, 2005). Mathews et al (1996) found depressed patients to have an attentional bias to socially threatening words using a dot-probe task, and Rinck and Becker (2005) found that depressed patients were more distracted by irrelevant depression-related words when undertaking a visual search task. Dot-probe tasks with emotional faces rather than words have also revealed negative biases in depressed patients (Gotlib et al 2004a, 2004b; Joormann and Gotlib, 2007). Some studies have failed to replicate negative attentional biases (Hill and Knowles, 1991; Neshat-Doost et al, 2000); however, as for the memory studies, the use of subclinical populations may be one significant cause of these discrepancies (Rinck and Becker, 2005).
Impairments of social and moral emotion
Feelings of inadequacy, self-blame, worthlessness, and hopelessness are core, specific, and distinctive symptoms of severe depression. Cognitive-behavioral psychotherapy models of depression (Beck et al, 1979), especially the revised learned helplessness model (Abramson et al, 1978) have pointed to the importance of this overgeneral self-blame. DSM-IV has included inappropriate feelings of guilt and worthlessness as characteristic symptoms of major depression with melancholic features (American Psychiatric Association, 2000). Excessive feelings of guilt were the second strongest predictor (after appetite disturbance) for a positive family history of major depression in a large family study (Leckman et al, 1984), and feelings of inadequacy or reduced self-worth in depression are seen across cultures (Sartorius et al, 1980). Pathological feelings of guilt were observed only in subsets of patients. Similarly, another study found 75% of inpatients with major depression to feel severe worthlessness and only 20% to have high levels of guilt (Prosen et al, 1983).
Whereas psychopathological interviews usually focus on self-reported feelings of guilt or self-reproach, more recent interest in shame as a depressogenic emotion has been raised by social psychologists. Both shame and guilt entail attributions of internal causal agency, but shame may be related to uncontrollable internal factors, such as character, whereas guilt in healthy people may be related to controllable internal causes (Weiner, 1985). Janoff-Bulman (1979) has distinguished between characterological and behavioral self-blame (Janoff-Bulman, 1979) and pointed to the depressogenic importance of the former, but not the latter. Guilt has been operationally defined and measured mostly as an adaptive form of behavioral self-blame and shame as the characterological form of blaming oneself in a maladaptive manner (Tangney, 1990).
Shame proneness, but not guilt proneness (as defined above), correlates with depressive symptoms in nonclinical populations (Tangney et al, 1992; Thompson and Berenbaum, 2006). Bodily shame was shown to have an important role in people with childhood abuse and was associated with chronic and recurrent outcomes in depressive disorders (Andrews, 1995). Perception of being externally shamed by others was correlated with depressive symptoms in a sample of patients with a clinical diagnosis of depression (Gilbert et al, 2009). In a large clinical study of patients with current major depression guilt, but not shame, measures were associated with the severity of depression (Alexander et al, 1999). In a comparably smaller clinical study in patients with major depression, both shame and guilt proneness were found to be increased (Ghatavi et al, 2002). Other researchers have shown that certain forms of guilt when operationalized as taking responsibility for other people's misfortune, even when one had no direct causal agency (survivor guilt), are increased in patients with major depression (O'Connor et al, 2002). Self-blaming tendencies in patients with major depression have also been demonstrated using a scale that includes items in which patients label their own feeling as ‘guilt’ or ‘shame’ (Berrios et al, 1992). Taken together, there is evidence that both shame and guilt are increased in patients with major depression when measured on self-report questionnaires. A direct comparison of studies is difficult because of differences in measures and constructs used. Guilt proneness seems to be associated with depressive symptoms in patients with current major depression only, but not in healthy people. Shame proneness in contrast may be associated with depressive symptoms in the absence of guilt only in people without major depressive disorders. One important shortcoming of investigations of guilt and shame is that clear distinctions between shame and guilt constructs and measures that are valid for the study of healthy populations may not be valid for patients.
Impairments of Affective Cognition in Remitted Depression and Vulnerable Individuals
Bhagwagar and Cowen (2008) suggested that studying unmedicated recovered depressed individuals could provide insights into trait abnormalities, which may underlie vulnerability to depression. This approach has gained considerable momentum in the last few years. Leppanen (2006) reviewed the literature on negative cognitive biases in remission and commented that although some biases do seem to persist, there is marked variation. Studies of attention and emotion perception have found reasonably consistent biases. For example, Atchley et al (2003) showed negatively biased processing of information in remitted depressed participants, whereas controls were positively biased. Joormann and Gotlib (2007) showed a similar pattern of biases using a dot-probe task with happy and sad faces. Bhagwagar et al (2004) reported that remitted patients showed greater recognition of fearful faces compared with controls, and Leppanen et al (2004) showed impairment in identifying neutral faces, similar to that seen in currently depressed patients. Our own unpublished data suggest increased recognition of negative face emotion in remitted patients due to altered response biases rather than discrimination. Other studies have found that remitted individuals display negative biases specifically in the context of a further manipulation. For example, negative biases in attention (McCabe et al, 2000) and emotional memory (Teasdale and Dent, 1987; Hedlund and Rude, 1995; Timbremont and Braet, 2004; Ramel et al, 2007) have been observed in remitted depression if a negative mood state is induced.
Negative biases in remitted patients suggest that these effects may represent trait vulnerability rather than a state-dependent phenomenon. However, findings in remitted patients could also reflect a ‘scar’ due to previous depression. Studies in never-depressed individuals at high risk provide support both for the former position from recent evidence finding negative processing biases (Chan et al, 2007; Joormann and Gotlib, 2007) and for the latter from no evidence of altered face emotion recognition accuracy (Le Masurier et al, 2007; Mannie et al, 2007), although these two studies did report subtle alterations in response latency. Studies in first-episode patients also offer some support for trait vulnerability; eg, adolescents with recent first-episode depression also show altered attentional biases (Kyte et al, 2005). This is an area that warrants further investigation as it has implications both for the mechanisms involved and for preventative interventions.
Summary
Behavioral studies of affective cognition in depression suggest that in a context of generalized performance deficits, negative biases can be observed in emotion categorization and bias tasks. These biases may manifest themselves as a frank bias toward negative material or an absence of a more positive bias observed in controls. It has been argued that these deficits at the interface between affect and cognition may be more specific to depression than generalized performance deficits (Clark et al, 2009; Chamberlain and Sahakian, 2006) and may represent an important target for treatment (Clark et al, 2009; Harmer et al, 2010). This account is in keeping with the importance of moral emotions in the pathophysiology of depression. Emotions such as guilt and shame are fundamentally cognitive emotions depending on cognitive modulation of basic emotional responses.
NEUROIMAGING OF AFFECTIVE COGNITION IN MOOD DISORDER
Processing Emotional Images
There have been relatively few studies using emotional IAPS pictures with fMRI in depression. For example, Wagner et al (2004) reported enhanced hippocampal response to positive pictures and enhanced amygdala, OFC, and PFC responses to negative pictures in patients. In the context of a treatment study, Davidson et al (2003) reported reduced insula and ACC responses to negative pictures in patients compared with controls, consistent with a finding of reduced ACC response to sad films (Beauregard et al, 1998). A different pattern of responses was reported by Anand et al (2005) who reported increased activation of ACC, insula, and parahippocampal areas in response to negative pictures in patients, as well as decreased cortico-limbic connectivity. Lee et al (2008) reported reduced activity in the right hippocampus and right insula to negative pictures, and reduced activity in the right ACC and left insula to positive pictures. Severity of depression correlated with responses to negative pictures, with no correlation for positive pictures. Clearly, there are marked discrepancies between studies which may reflect differences in patient characteristics or paradigms. Evidence that task factors may be critical comes from a recent study indicating that responses to emotional pictures are differentially modulated by expectation in depressed patients compared with controls (Bermpohl et al, 2009).
There have also been a number of studies looking at perception of emotional valence in words. Siegle et al (2002, 2006, 2007) used self-referential emotional words and found that amygdala response to negative words was both increased and sustained for significantly longer in depressed patients compared with controls. Kumari et al (2003) used combined pictures and sentences to create a positive, negative, or neutral message. In response to negative (compared with neutral) stimuli, they found that treatment-resistant depressed patients showed decreased response in the ACC. For positive stimuli, patients showed decreased response in the left medial frontal gyrus/ACC and hippocampus.
Face Processing
Neuronal responses to emotional faces in depression have been extensively studied. Some fMRI studies have reported enhanced amygdala response to faces in patients relative to controls (Sheline et al, 2001; Fu et al, 2004; Surguladze et al, 2005; Fu et al, 2008a, 2008b), but others have failed to replicate this finding (Gotlib et al, 2005; Keedwell et al, 2005; Dannlowski et al, 2007; Lee et al, 2008) and one study (Lawrence et al, 2004) reported reduced amygdala response to emotional faces in depression. These studies also report group differences in a range of other areas, including the hippocampus, hippocampal gyrus, cingulate gyrus, insula, fusiform gyrus, caudate, thalamus, ventral striatum and frontal, and parietal and temporal regions, using happy, sad, and fearful faces. However, the regions reported for each emotion, and the direction of group differences, vary from study to study. These inconsistencies may be partly attributable to methodological factors such as overt compared with covert presentation, and whether backward masking is used (Sheline et al, 2001; Dannlowski et al, 2007). Medication status may also be a significant confound, with some studies using medicated participants (Lawrence et al, 2004; Fu et al, 2008a, 2008b; Gotlib et al, 2005), some unmedicated (Sheline et al, 2001; Fu et al, 2004, 2007; Surguladze et al, 2005), and some mixed medication status (Gotlib et al, 2005; Keedwell et al, 2005; Lee et al, 2008).
Affective Biases
Versions of the face emotion recognition paradigm incorporating a memory component have also been developed. Roberson-Nay et al (2006) compared neural responses to faces that were subsequently remembered to those that were forgotten. They found that although depressed participants exhibited poorer memory overall, they showed greater left amygdala activation in response to faces (regardless of emotion) that they subsequently remembered than to those they forgot. This suggests that the depth of processing may be a critical factor in determining neuronal response. By contrast, Hamilton and Gotlib (2008) reported that depressed patients showed enhanced right amygdala activity to negative but not positive emotional pictures that were subsequently remembered, as well as enhanced connectivity with the hippocampus. Memory for negative pictures was also enhanced. In spite of the discrepancies between these studies, both are consistent with a hypothesis that amygdala hyperactivity at encoding could be a basis for negatively biased memory. More generally, Phillips et al (2003) argued that limbic overactivity during initial evaluation of emotional stimuli, combined with a failure of cortical control (reflected by prefrontal hypoactivity), cause negative biases observed in depression.
Neuroimaging has also been used to assess the neuronal basis of negative attentional biases. Mitterschiffthaler et al (2003) used an emotional Stroop task and showed that behavioral bias toward negative stimuli in anhedonic patients was associated with enhanced ACC response. Similarly, Elliott et al (2002) reported increased ventral ACC response to sad targets and decreased response to happy targets in an affective Go/No-Go task in depressed patients, the reverse of the pattern observed in controls (see Figure 3). In this task, sad distracters were associated with enhanced OFC response in depressed patients, which we interpreted as reflecting the inability to inhibit instinctive responses to mood-congruent information. Enhanced inferior frontal responses associated with the inability to disengage from sad material have also been observed by Wang et al (2008) and Dichter et al (2009). Biases of attention may reflect either impairments of top-down cognitive control over affective responses or enhanced bottom-up responses to affective stimuli (or potentially a combination of the two). Our findings (Elliott et al, 2002) tentatively suggest that both mechanisms may be involved at a neuronal level in depression. Fales et al (2008) addressed this issue explicitly using an attentional interference task with emotional distracters to test for top-down vs bottom-up dysfunction. Depressed patients showed enhanced amygdala response to unattended fear-related stimuli. By contrast, control participants showed increased activity in the right DLPFC when actively ignoring fear stimuli, which patients with depression did not show. These results provide empirical support for the theory that both top-down and bottom-up mechanisms are important in attentional biases in depression.
The affective Go/No-Go task in depression. The three panels show behavioral responses to the task (adapted from Erickson et al, 2005) and the ventral ACC focus mediating mood-congruent bias, with enhanced response to happy targets in controls and sad targets in patients (adapted from Elliott et al, 2002).
Social and Moral Emotion
The frontopolar and subgenual cingulate cortex, which have been linked to the experience of guilt in healthy participants (see above), are important nodes in a network of brain regions that have been implicated in the pathogenesis of major depression (Seminowicz et al, 2004; Ebert and Ebmeier, 1996; Drevets and Savitz, 2008; Mayberg, 2007). However, to our knowledge, studies directly investigating the neural correlates of these specific moral emotions in patients with major depression are lacking so far.
Connectivity/Neuronal Models of Affective Cognition in Mood Disorder
In spite of the complexities of the literature on neuroimaging affective cognition in depression, it is clear that structures involved in normal affective cognition are functionally abnormal in depression. Disrupted responses of limbic regions, particularly the amygdala, have been associated with biases observed in depressed patients toward negative information, and the ventral ACC and other ventromedial regions represent an important neural interface between cognitive and emotional processing. Disrupted cortico-limbic circuits, with a key modulating function for the ventral ACC, may explain both emotional biases and cognitive deficits in depression, as suggested in an influential model initially proposed by Mayberg (1997, 1999). This model has subsequently been developed and shown to have value in both diagnosis and prediction of treatment response (Mayberg, 2002, 2003; Ressler and Mayberg, 2007) (see Figure 4). Network models of depression can be tested explicitly using connectivity analyses.
Mayberg's network model of depression. va=ventral anterior, dp=dorsal posterior. Regions within each grouping (‘compartment’) are heavily interconnected and connections between compartments are indicated by black errors. Figure adapted from Mayberg (2009), which also proposes that different therapeutic interventions may differentially target particular compartments and connections.
Several studies have explored functional coupling of the amygdala and prefrontal regions (Johnstone et al, 2007; Siegle et al, 2007; Chen et al, 2008; Matthews et al, 2008). Matthews et al (2008) reported reduced functional coupling of the amygdala and supragenual ACC during emotion processing that increased with severity of depression. A more comprehensive approach to connectivity is to use techniques such as structural equation modeling and dynamic causal modeling (DCM) to explore changes in a prespecified network of regions. Using this approach, we observed abnormal connectivity associated with sad face processing in remitted depression (Goulden et al, 2010). Almeida et al (2009) reported disrupted OFC–amygdala connectivity in response to happy faces and a nonsignificant trend toward the same effect of sad faces. Frodl et al (2010) reported reduced OFC connectivity during emotional processing in never-medicated patients. Although there is a need for considerable further research, it seems that connectivity analysis exploring functional disruption within emotional networks may provide a crucial tool for exploring the affective-cognitive interface in depression.
Neuroimaging of Affective Cognition in Remitted Depression and Vulnerable Individuals
Despite the growing literature highlighting persistent emotional biases in remitted depression, relatively few studies have investigated the neural correlates of these findings. Liotti et al (2000) demonstrated that when a sad mood was induced, rCBF changes in remitted depressed participants were similar to those seen in current depression, with decreases observed in the medial OFC, extending to the anterior medial PFC, and the anterior thalamus, and increases in the VLPFC and lateral OFC. Gemar et al (2007) found a similar pattern of results in unmedicated remitted participants, indicating that these results cannot be explained as medication effects. Ramel et al (2007) also used sad mood induction and found that although sad, amygdala response to negative self-referential words predicted the subsequent proportion of negative words recalled in remitted participants. There is also neuroimaging evidence for persistent abnormalities in depression even in the absence of induced depressed mood. Neumeister et al (2006) demonstrated rCBF to be enhanced in the amygdala and reduced in the left ventral striatum in remitted participants viewing sad faces.
Deficits in remitted depression are suggestive of trait vulnerability; however, it is also possible that the deficits represent a ‘scarring’ effect of acute depressive episodes. Studies with never-depressed subjects at high risk are helpful in clarifying this issue. Van der Veen et al (2007) and Monk et al (2008) have reported abnormal amygdala responses to negative facial expression in people at risk of depression on the basis of family history. Similarly, people vulnerable to depression through the personality trait of neuroticism show enhanced amygdala responses to fearful faces (Chan et al, 2009), disrupted coupling of the amygdala and ACC in response to emotional faces (Cremers et al, 2010), and abnormal patterns of the ACC response during categorization and memory of negative words (Chan et al, 2008). Recent data from our research suggest that personality traits conveying vulnerability (rumination) may interact with depression history to produce trait-related changes in limbic responses to emotional faces (Thomas, 2009).
ROLE OF MONOAMINES IN AFFECTIVE COGNITION
Role of Monoamines in Affective Disturbance
Introduction: rationale for focus on 5-HT
Nearly all commonly used antidepressant drugs work by enhancing 5-HT neurotransmission. No new principle of action has emerged in the half century since they were discovered: monoamine oxidase inhibitors in 1952 and monoamine reuptake inhibitors in 1957. Early drugs had many pharmacological actions causing side effects and this led to the development of selective serotonin reuptake inhibitors (SSRIs), which are now the most widely used antidepressants. That SSRIs function by enhancing synaptic 5-HT availability was decisively established by the technique of acute tryptophan depletion (ATD). This ingenious experimental method involves administration of an amino-acid drink without the essential amino-acid precursor of 5-HT, tryptophan, and typically leads to a 70–90% reduction in plasma tryptophan concentration over the next 4–6 h which results in reduced CNS 5-HT synthesis, turnover, and neurotransmission (Hood et al, 2005). ATD in patients recently recovered from depression induces a relapse of symptoms (Delgado et al, 1994; Ruhe et al, 2007). Importantly, ATD reinstates the depressive cognitions and preoccupations that characterized their illnesses rather than imposing a different depressive experience. ATD is much less effective in inducing depressed mood once recovery is established for several months, and it has little effect on mood in healthy people with no familial risk or previous history of depression (Ruhe et al, 2007). However, as reviewed below, ATD can induce changes in emotional processing without overt mood change. These findings lead to three inferences: (1) SSRIs induce recovery by enhancing 5-HT function; (2) impaired 5-HT function may not be a sufficient condition for depression, implicating other vulnerability factors; and (3) enhanced 5-HT function triggers downstream changes, such as formation of new forebrain connections or resetting of central control of stress hormone secretion, which maintain remission.
5-HT function in depression
The 5-HT hypothesis of antidepressant action soon generated its corollary: that depression involves deficient 5-HT function. Many studies in the 1960s and 1970s measured peripheral indices of central 5-HT function such as cerebrospinal fluid 5-hydroxyindole acetic acid with contradictory results. Perhaps the most consistent abnormality was reduced circulating concentrations of tryptophan. In the 1980s, neuroendocrine drug challenge techniques were developed in which peripheral hormone responses to administration of drugs with known central actions were used as a measure of dynamic CNS (hypothalamic) transmitter function. Intravenous citalopram (an SSRI) and tryptophan challenge were found reasonably consistently to evoke reduced prolactin and growth hormone responses in depressives compared with controls (Cowen and Charig, 1987; Deakin et al, 1990; Price et al, 1991, Kapitany et al, 1999). These drugs both act through presynaptic 5-HT neurons and this would be compatible with impaired 5-HT neuronal function in depression.
Hopes that in vivo direct quantification of 5-HT receptors and uptake sites using positron emission tomography (PET) would pinpoint that the mechanisms of impaired 5-HT function in depression have not yet been realized, with no clear consensus from studies so far. Some studies have reported a higher density of transporters in depressed patients (Cannon et al, 2007), whereas others have reported lower density (Selvaraj et al, 2010). A recent series of studies (Meyer et al, 2006) have suggested a possible resolution to these discrepancies by arguing that impaired 5-HT function in depression may come about through depletion of synaptic 5-HT by increased amounts of the intraneuronal enzyme mono-amine oxidase A. The authors have evolved a novel conceptual reorientation (the advanced monoamine hypothesis) that the density of monoamine transporters modulates the degree to which synaptic monoamines, through the reuptake mechanism, are exposed to increased intraneuronal MAO. Further testing of this hypothesis is required.
Regardless of these debates, it is clear from PET studies that 5-HT function is disturbed in depression. There is also clear evidence that 5-HT function in depression is related to affective cognition. A number of PET studies in depression and healthy controls report associations of 5-HT biomarkers with affective cognition. For example, levels of 5-HT2A and 5-HT transporter (5-HTT) binding were found to be positively correlated with scores on the DAS (Meyer et al, 2003, 2004; Takano et al, 2007; Frokjaer et al, 2008; Bhagwagar et al, 2006). The inference is that low synaptic 5HT content, caused by increased uptake and indexed by upregulated 5HT2A receptor binding, negatively colors the perception of self, life, and the future. That this may be a surprisingly direct relationship and mediated by decreased 5-HT2A receptor stimulation is suggested by one study in healthy volunteers in which DAS scores decreased 1 h after acute administration of fenfluramine, a drug which releases 5-HT with effects mediated by 5HT2 receptors (Meyer et al, 2003).
In summary, although we do not dispute that other neurotransmitters may be important, there is a sound rationale for focusing on 5-HT in modulating disturbances of affective cognition in mood disorders. Furthermore, in vivo studies of 5-HT far outnumber those of other neurotransmitters. Within the limited scope of a review, it is not possible to consider all candidate neurotransmitter mechanisms.
Investigating the Effects of 5-HT on Affective Cognition
This section is primarily concerned with the effect of experimental manipulation of 5-HT neurotransmission on affective cognition using pharmacological challenges. However, there have also been an increasing number of studies investigating the effect of 5-HT genotype on affective processing and we briefly consider them in this review. Methodological considerations that need to be taken into account in interpreting pharmacological studies are summarized in Table 1. The most widely used technique to investigate 5-HT function has been ATD (see above), which reduces brain 5-HT neurotransmission. ATD can lead to temporary lowering of mood, which is a potential confound in studying affective processing; however, this seems confined to vulnerable individuals such as those with previous personal or family history of depression (Ruhe et al, 2007; Mendelsohn et al, 2009). Increasing brain 5-HT function has been studied less comprehensively and is achieved by either administration of tryptophan or a serotonergic antidepressant drug, most commonly, the SSRI citalopram. A complication arises in interpreting studies using repeated administration of antidepressant drugs in which, although clinically relevant, the neurochemical effects are likely to be complex. In patient treatment studies, and owing to the uncertain neurochemical effect of repeated administration, there are also changes in mood state which alter affective processing.
5-HT Genotype and Affective Processing
The most commonly investigated 5-HT genotype has been the 5-HTT-linked promoter region (5-HTTLPR) gene which is usually reported in relation to its short (S) and long (L) alleles, although other variations exist. L alleles result in greater mRNA transcription and more active 5-HTT function (Parsey et al, 2006) and have been associated with a better treatment response to SSRI antidepressants, although discrepant findings exist (Serretti et al, 2007).
Behaviorally, no effect of 5-HTTLPR genotype was observed on recognition of fearful faces (Marsh et al, 2006), although an interaction with ATD was found (see below). Affective bias tasks have shown more significant effects. Compared with LL homozygous, S-allele carriers have been reported to have an increased attentional bias for anxious, but not dysphoric word stimuli (Beevers et al, 2007), greater difficulty in disengaging from sad, happy, and fearful stimuli (Beevers et al, 2009b), a lack of the bias toward positive affective pictures seen with LL homozygotes in a dot-probe paradigm (Fox et al, 2009), increased selective attention toward fear-related pictures (spiders) in women (Osinsky et al, 2008) and in children, and attentional bias toward sad but not happy or angry faces, which interacted with a maternal history of depression (Gibb et al, 2009). In contrast, a study using an eye-tracking task reported that S-allele carriers, but not LL homozygotes, had a bias away from negative toward positive scenes (Beevers et al, 2009a). Not all studies of affective bias have reported effects; 5-HTTLPR status had no effect on an affective Go/No-Go task (Roiser et al, 2007) or verbal emotional memory (Roiser et al, 2007; Strange et al, 2008). Similarly, a promoter polymorphism of the tryptophan hydroxylase 2 gene (TPH2), which regulates 5-HT synthesis in the brain, did not affect the primary interference outcomes in an emotional Stroop paradigm (Osinsky et al, 2009). It is possible that intervening variables are important in determining whether effects are observed. For example, Lemogne et al (2009) reported that S-allele carrier status had no direct effect on an emotional memory task, but interacted with stressful life events to produce significant emotional biases.
In contrast to behavioral studies, fMRI has shown effects of S-carrier status on the neuronal basis of emotion recognition. S carriers have been reported to have increased amygdala activation to fearful faces or threatening stimuli in most studies, and a meta-analysis of 14 studies concluded that it might account for up to 10% of the variance (Munafo et al, 2008). In studies published since the meta-analysis, there have been further positive (Furmark et al, 2009; Lau et al, 2009; Williams et al, 2009) but also negative (Demaree et al, 2009; Friedel et al, 2009) findings. Polymorphisms of the TPH2 gene have been reported to have independent (Brown et al, 2005; Canli et al, 2005) and additive effects with the 5-HTTLPR genotype (Canli et al, 2008; Furmark et al, 2009) on amygdala activation. A further study has found increased activation for sad faces with the TPH1 gene A218C polymorphism (Lee et al, 2009). Finally, a common functional polymorphism of the 5-HT1A gene has been associated with decreased amygdala response to angry and fearful faces (Fakra et al, 2009). In connectivity studies, the S allele has been associated with increased medial prefrontal-amygdala connectivity to negative pictures (Friedel et al, 2009), weaker coupling between the perigenual cingulate and amygdala to fearful and angry faces (Pezawas et al, 2005), and greater functional connectivity between the right amygdala and the fusiform gyrus and the right fusiform gyrus and the right VLPFC to fearful faces (Surguladze et al, 2008).
In summary, there is an impressive weight of evidence to support altered affective cognition with 5-HT genotypes. In behavioral studies, the S allele of 5-HTTLPR appears to be linked to altered affective bias, whereas the balance of fMRI evidence suggests increased amygdala reactivity to perception of negative emotional stimuli and altered connectivity. These studies provide strong support for 5-HT neuronal makeup influencing affective cognition, presumably through developmental influences, although discrepancies between studies suggest that intervening variables such as life stress may also be crucial. It is also important to recognize that genotype does not seem to affect 5-HTT availability (Parsey et al, 2006) and is not a measure of dynamic 5-HT function for which studies of 5-HT manipulation are required.
The Effect of Manipulating 5-HT on Affective Cognition: Behavioral Studies
A recent systematic review including 66 studies on the effects on cognitive function of decreasing central 5-HT function with ATD concluded that there were only very modest effects on tests of ‘cold’ cognition (ie, tests requiring cognitive evaluation of stimuli without emotional content; Mendelsohn et al, 2009). Given the role of 5-HT in affective disorder, and the effects of 5-HT genotype on affective cognition, it is plausible that affective (or ‘hot’) cognitive tasks may be more sensitive to 5-HT manipulation.
Findings in healthy volunteers: processing emotional images and faces
Recognition of emotion in pictures has not been widely studied; one study found no effect of combined decreasing of plasma tryptophan, phenylalanine, and tyrosine on valence or arousal ratings of IAPS in female volunteers (Hughes et al, 2004) and a further study requiring participants to choose between different intensities of an emotion without explicitly identifying the emotion found no effect of ATD (van der Veen et al, 2007).
Behavioral studies of face emotion recognition have used face emotions presented at differing degrees of intensity. Most studies of 5-HT manipulations find some effect but the picture is not fully consistent, reflecting several factors, including a pharmacological mode of action. Of three studies using ATD to decrease 5-HT function, two found a decrease in fear recognition with no effect on recognizing other emotions; in one study, it occurred in females but not males (Harmer et al, 2003c) and the other only in S-allele carriers of the 5-HTT (Marsh et al, 2006). The other study, using low-dose ATD, found no effect on fear recognition but an increase in happiness recognition (Hayward et al, 2005). Increasing 5-HT function acutely in five studies of healthy volunteers from the same group in Oxford, using a single dose of tryptophan or citalopram, increased fear recognition in four studies (Attenburrow et al, 2003; Bhagwagar et al, 2004; Browning et al, 2007; Harmer et al, 2003a) but not in the fifth (Murphy et al, 2009a). In three of the studies, there was also an increase in recognition of happiness (Attenburrow et al, 2003; Harmer et al, 2003a; Murphy et al, 2009a).
Drugs with more complex effects at 5-HT receptors, as well as other neurotransmitter receptors, have also been studied. Duloxetine, which inhibits both 5-HT and noradrenaline reuptake, failed to increase fear recognition (Harmer et al, 2008), but increases in happiness and disgust recognition were found. Acute treatment with the antidepressant mirtazapine, which has multiple effects on 5-HT and noradrenaline function, decreased rather than increased fear recognition with no effect on other emotions (Arnone et al, 2009). It is possible that noradrenergic effects with duloxetine and mirtazapine, or 5-HT2 receptor blockade with mirtazapine, might partly explain the discrepant results. Finally, acute treatment with the 5-HT3 antagonist, ondansetron, failed to alter face emotion recognition (Harmer et al, 2006b), potentially suggesting that this receptor is of limited importance in this context. Chronic administration of 5-HT manipulations in healthy volunteers has been undertaken much more rarely. Repeated administration of tryptophan (Murphy et al, 2006) decreased recognition of disgust but increased recognition of happiness. Repeated citalopram (Harmer et al, 2004) decreased recognition of disgust, surprise, and fear. As discussed above, adaptive effects after repeated drug administration preclude a simple interpretation.
Findings in healthy volunteers: emotional memory bias
A series of studies from the Oxford group have used an emotional categorization task requiring identification of self-referential trait adjectives as positive or negative and then having a surprise memory recall test after a short delay. The main outcomes are speed of categorization and correct recall related to valence. No effects of increasing 5-HT function were found using a single dose of citalopram (Browning et al, 2007), subchronic tryptophan (Murphy et al, 2006), or acute duloxetine (Harmer et al, 2008), although with duloxetine, there were more falsely remembered (intrusive) positive words. 5-HT3 antagonism with ondansetron also had no effect (Harmer et al, 2006b), but a single dose of mirtazapine did increase the proportion of positive items recalled (Arnone et al, 2009), possibly attributable to its noradrenergic effects as a similar result was found with acute reboxetine, an antidepressant which blocks noradrenaline reuptake (Harmer et al, 2003b). Consistent with these generally negative findings, ATD had no effect in a similar task studying selective recall of emotional words (Roiser et al, 2008). However, in another study, ATD resulted in slightly fewer positive words being recognized in a memory task in which participants had to assign positive or negative feelings to neutral words during encoding (van der Veen et al, 2006). Two studies investigated repeated administration of serotonergic agents on the emotion categorization task from the Oxford group found no effect of a week of tryptophan treatment (Murphy et al, 2006), but 7 days of citalopram treatment resulted in a trend to faster categorization and an increase in the recall of positive compared with negative traits. The lack of effect of acute 5-HT manipulation or repeated tryptophan administration suggests that secondary adaptive effects, not necessarily involving 5-HT, might be involved in the effects of citalopram in the latter study.
These predominantly negative studies all used short delays before recall, and the situation may be different with longer delays during which memory decay occurs. In one study using an affective version of the Rey Auditory Verbal Learning Test, there was generally impaired recall under ATD, most strongly for neutral words after 30 min, but after 18 h, there was less forgetting of negative compared with positive and neutral words (Klaassen et al, 2002). Using the Cahill task, we similarly found participants who received ATD had generally impaired recall after 1 week but with relative sparing of unpleasant compared with neutral scenes (Richell and Anderson, 2001). Therefore, one possibility is that shorter-term emotional memory is not greatly influenced by 5-HT, but the rate of memory decay for negative information over longer periods is more sensitive. This may have relevance for relative sparing of memory for past negative events seen in depression.
Findings in healthy volunteers: attentional bias
Three studies using the emotional Stroop found no effect of ATD or combined tryptophan, phenylalanine, and tyrosine depletion on reaction times (Evers et al, 2009; Hughes et al, 2004; Munafo et al, 2006). Although one study reported that ATD increased errors on negative words in those without, but not with, a family history of depression (Evers et al, 2009). Similarly four out of five studies found no overall effect of ATD on reaction times in the affective Go/No-Go task (Firk and Markus, 2008; Robinson and Sahakian, 2009; Roiser et al, 2008; Rubinsztein et al, 2001); one study did find that ATD prevented the baseline faster reaction times to happy targets, consistent with a negative bias caused by ATD (Murphy et al, 2002). Two of the overall negative studies found subtle effects; slower responses to happy targets with ATD in those with a positive family history of depression (Firk and Markus, 2008) and a reduction by ATD of commission errors to happy distractors (Robinson and Sahakian, 2009). In a dot-probe paradigm, citalopram decreased the reaction time when the probe replaced a positive rather than neutral word which was not seen on placebo, although no effect was seen with negative words (Browning et al, 2007). Using a similar task, but with fearful, happy, and neutral faces, 7 days of citalopram treatment had no effect on reaction times associated with happy compared with neutral faces but abolished the bias toward fearful faces (measured by faster reaction times) seen on placebo (Murphy et al, 2009b).
In summary, in healthy volunteers, there is a possible acute facilitatory effect of 5-HT on fear recognition, and less consistently happiness recognition, in women; the effect in men is less clear. The effect of chronic treatment with 5-HT agents may result in different alterations in emotion recognition compared with acute manipulations, shifting the balance toward positive and away from negative emotions. 5-HT does not seem to selectively alter short-term encoding or recall of emotional material but may facilitate longer-term forgetting of negative events. The balance of evidence suggests that 5-HT has a subtle role in affective cognition; further research is required to characterize these effects more fully and disambiguate effects at different receptors.
Findings in patients
There have been few behavioral studies in patients. Patients with GAD had increased negative bias to ambiguous homophones compared with controls which decreased with SSRI treatment (Mogg et al, 2004); the change in bias correlated with clinical improvement suggesting a mood rather than pharmacological effect. A mood effect is consistent with the lack of effect of citalopram in healthy volunteers (see above). We found increases in both negative and positive face emotion recognition after 6 weeks treatment in depressed patients randomized to citalopram or reboxetine (Tranter et al, 2009); none of these changes correlated with clinical outcome and there was no difference between drugs. However, patients tended to improve more on citalopram and improvement in recognition of happiness at 2 weeks predicted clinical outcome at 6 weeks, accounting for 21% of the variance. The lack of a control group makes interpretation difficult, but, consistent with the literature, we found that drug-free patients show decreased discrimination of face emotions, which was not present in those on antidepressants (IMA unpublished data). This supports there being independent mood and drug effects on face emotion recognition.
Findings in remitted patients
Bhagwagar et al (2004) found that drug-free remitted depressed women had more accurate recognition of fearful faces, but not other emotions, compared with controls and this decreased (‘normalized’) after a single dose of citalopram, opposite to the increased recognition seen in controls (discussed above). In partial support of this, we also found an increase in bias to identification of face emotions (but not confined to fear) in unmedicated individuals with remitted depression, which was not present in those on antidepressants (IMA unpublished data). However, in direct contrast, a study in medicated remitted depressed patients (without a control group) found that decreasing 5-HT function with high-dose, compared with low-dose, ATD selectively decreased fear recognition (as has been found in healthy controls) (Merens et al, 2008). In the same study, there was no effect of ATD at either dose on an affective Go/No-Go task or an emotional implicit association task. Roiser et al (2009) also reported no effect of ATD on the affective Go/No-Go task. A study using an emotional Stroop task in medicated and unmedicated remitted depressed patients found that there was no effect of low-dose ATD on mood or emotional Stroop interference in unmedicated participants (or controls) but slower color naming of negative words compared with neutral words in remitted depressed patients on antidepressants (Munafo et al, 2006). However, the slight increase seen in depressed mood in the latter group is a potential confound.
In summary, affective cognition seems to ‘normalize’ with antidepressant treatment in depressed and anxious patients, but the role of 5-HT in these improvements is not clear. The limited evidence broadly suggests that remitted depressed patients may not differ from controls in 5-HT modulation of affective processing biases.
The Effect of Manipulating 5-HT on Affective Recognition: Imaging Studies
Findings in healthy volunteers: face processing
PET with serotonergic ligands has been used to explore relationships between 5-HT function and fMRI responses to various face processing tasks. A recent study has shown that left amygdala 5-HTT availability measured using PET imaging of [11C]-DASP binding correlated inversely with left amygdala fMRI activation in an emotion face matching task (Rhodes et al, 2007). Other studies, using the same task, have found that dorsal raphe nucleus 5-HT1A autoreceptor-binding potential, measured with [11C] WAY100635, correlated inversely with bilateral amygdala fMRI activation (Fisher et al, 2006) and that medial PFC (mPFC) 5-HT2A receptor density, measured using [18F]-altanserin, correlated negatively with right amygdala fMRI activity and positively with mPFC–amygdala functional coupling (Fisher et al, 2009). These data, along with the genetic data described above, support a role for 5-HT neurons in modulating amygdala reactivity to emotional stimuli but cannot directly determine the functional dynamic effects of 5-HT.
To address this question, studies have used modulation pharmaco-MRI (Anderson et al, 2009), exploring the effects of a 5-HT manipulation on functional response to emotion recognition. Results of these studies are difficult to synthesize into a simple story. With ATD, an increase in right amygdala activation to emotional faces irrespective of valence has been found in two studies (Fusar-Poli et al, 2007; van der Veen et al, 2007) but decreased left amygdala activation was found in one of these (Fusar-Poli et al, 2007). A third study reported no effect (Cools et al, 2005), but did find an interaction between the personality trait of threat sensitivity and positive modulation by ATD of right amygdala activation. Studies acutely enhancing 5-HT function with citalopram have found decreased amygdala responses to negative compared with neutral faces (Anderson et al, 2007; Del-Ben et al, 2005; Murphy et al, 2009a), but increased activation comparing faces (emotional and neutral) and a sensorimotor control task (Bigos et al, 2008). A study using unpleasant compared with pleasant pictures found that citalopram decreased left amygdala activation (Takahashi et al, 2005). Consistent effects have not been found in other brain regions, although in two studies, viewing negative emotions had opposite effects on the right VLPFC of decreasing 5-HT function with ATD and increasing it with citalopram (leading to increased and decreased activation, respectively) (Del-Ben et al, 2005; Fusar-Poli et al, 2007).
Repeated administration of citalopram or escitalopram for 1–3 weeks has been reported to decrease amygdala activation to fearful faces in two studies (Arce et al, 2008; Harmer et al, 2006a), but on different sides, with no effect in a third (Norbury et al, 2009), which instead found enhanced amygdala responses to happy faces. In one study, reduced mPFC response to fearful faces was also seen (Harmer et al, 2006a). Therefore, for both acute and chronic 5-HT administration, there are inconsistencies in the literature. This may be partially attributable to differences in task instructions and cognitive demands that have been shown to significantly influence both amygdala and VLPFC responses (Lieberman et al, 2007). If, as argued above, effects of 5-HT manipulations in healthy volunteers are subtle, such differences may be critical.
Findings in healthy volunteers: affective bias
A study of affective processing bias as measured by the emotional Stroop did not find any alteration in neuronal activation with ATD in women with or without a family history of depression (Evers et al, 2009; Evers et al, 2006). By contrast, in an elaborately analyzed study using the affective Go/No-Go task, Roiser et al (2008, 2009) found that ATD resulted in altered responses in various areas including enhanced activation of the amygdala and hippocampus on the left and the thalamus, anterior insula, and parahippocampal gyrus on the right for emotional compared with neutral targets, whereas decreased activity was seen in the ACC. Few changes were seen between happy and sad targets.
To summarize, the imaging evidence in healthy volunteers taken as a whole supports a role for 5-HT in the neuronal basis of affective cognition, particularly emotion categorization with insufficient evidence to draw conclusions about affective bias. Inconsistencies in the literature may reflect critical differences in paradigms used, and differences between different pharmacological manipulation protocols.
Findings in patients
As discussed above, treatment studies in depressed patients are difficult to interpret because of mood improvement, adaptive effects to repeated administration of antidepressants, and order effects. Treatment with the dual serotonin and noradrenaline reuptake inhibitor venlafaxine (Davidson et al, 2003) led to increased activation in the left insula cortex within 2 weeks in depressed patients compared with a decrease over time seen in controls when viewing negative relative to neutral pictures with a similar pattern seen by 8 weeks in the ACC. Although patients had lower activation of the ACC at baseline compared with controls, those with relatively greater activation had better symptomatic outcomes at 8 weeks. Fu et al (2004, 2007) treated depressed subjects with fluoxetine for 8 weeks with administration of an implicit face emotion fMRI task before and at the end of treatment with controls scanned at the same interval. Results are reported in separate publications for sad vs neutral faces (Fu et al, 2004) and for happy vs neutral faces (Fu et al, 2007). At baseline, patients had increased activation compared with controls to sad faces in a range of areas including the amygdala, hippocampus, caudate, insula, and ACC with no difference seen for happy faces. Patients had an increased dynamic range (the difference in activation between low- and high-intensity emotion) in the ACC and decreased dynamic range in the lateral PFC to sad faces, and a decreased dynamic range to happy faces in the bilateral thalamus and hippocampus. Over time, depressed patients showed a reduction in activation to sad faces in the left amygdala, which correlated with an increase in dynamic range in the left lateral PFC. Changes in activation to happy faces with treatment occurred in the posterior cortical areas, including visual-processing regions. Symptomatic improvement was associated with reduction in dynamic range to sad faces in the pregenual ACC and an increase in activation to happy faces in the left hippocampus and extrastriate regions. Schaefer et al (2006) studied depressed patients before and after treatment with venlafaxine and controls scanned twice with an average of 22 weeks between scans. A complex pattern of neuronal changes to different emotional pictures was observed, interpreted as a bias away from processing positive social stimuli in depression which improved with treatment.
In a study of an emotion interference task requiring identity judgments of pairs of faces (Fales et al, 2009), depressed patients showed a number of differences from controls including activating the left amygdala more than controls when fearful faces were presented as distractors, but less than controls when they were targets. After 8 weeks of treatment with various SSRIs, these differences normalized; other areas where there were significant differences between patients and controls did not show significant changes over time in patients, except for a decreased activation to fearful face distractors in the right DLPFC which increased with treatment. Recent evidence suggests that connectivity changes may underpin treatment effects on affective cognition. Chen et al (2008) described increased amygdala–ACC coupling during face emotion recognition as symptom severity reduced in response to antidepressant treatment.
In none of these studies is it possible to tease out pharmacological from mood-dependent changes in affective processing, given the lack of placebo treatment of patients or drug treatment of controls. As discussed above, remitted depressed patients offer an opportunity to investigate trait/scar differences in depression while minimizing mood confounds. In a study using an affective Go/No-Go task, the effect of decreasing 5-HT function using ATD was compared in drug-free remitted depressed women and controls (Roiser et al, 2009). Remitted depressed subjects differed significantly from controls with no increase in activation to emotional compared with neutral targets but an increased, rather than decreased, response to emotional distractors in the dorsal ACC. This area has been implicated in adjustment to conflict and error correction and may suggest altered 5-HT control of neurons in the region in response to extraneous emotional information. Further studies are required in remitted patients and in those vulnerable to depression to identify which aspects of affective processing may be sensitive to 5-HT manipulations.
Summary
5-HT effects on affective function in healthy controls are subtle at a behavioral level and seem to depend critically on both the exact manipulation and the task used. fMRI results more consistently support the view that 5-HT modulation affects normal affective cognition, indicating that there may be neuronal changes in the absence of measurable performance effects. Both behavioral and fMRI results broadly support an effect of 5-HT manipulation on affective cognition in depression; however; these results are typically confounded by changes in the depressed state in response to 5-HT modulation. Most frequently, studies using tasks involving the perception or recognition of face emotions have been used and the results are summarized in Table 2, illustrating limitations and gaps in the data set.
CONCLUSIONS
In this review, we considered affective cognition, its disruption in depression, and the possible modulatory role of 5-HT. Given the large and ever-increasing literature in this area, we had to be selective but the evidence that we reviewed leads to certain important conclusions.
The neuronal basis of affective cognition depends on a network of brain regions. Neuropsychological studies highlight roles of the amygdala (particularly in the recognition of emotion and emotional memory) and VMPFC (particularly in social and moral emotions). The neuroimaging literature in healthy volunteers confirms the importance of these regions as part of a wider network also including the hippocampus, parahippocampal gyrus, temporal cortices, insula, ACC, and other medial and ventral prefrontal regions. The pattern of responses within these networks is dependent on specific emotions, with more emphasis on limbic regions for basic emotions, such as fear, and with more emphasis on medial prefrontal regions for complex emotions, such as guilt. The pattern also depends on task demands, with emotion perception tasks preferentially engaging posterior and subcortical components of the network and prefrontal regions becoming more involved as more integration with cognitive processes is required. Influential models of affective processing have been proposed, involving a posterior/subcortical system for emotion perception and a prefrontal system exerting regulatory control (Ochsner and Gross, 2005; Phillips et al, 2003, 2008). Although we have not discussed reward learning or emotional decision making in this study, similar models can be derived to describe these processes (Dolan, 2007). Such interconnected models of affective cognition allow for both top-down control of emotional response by cognitive mechanisms and bottom-up biases of cognitive processing by emotional appraisal; however, affective cognition may ultimately be better explained by a more integrative model. Inconsistencies and gaps in the current literature must be addressed before a comprehensive model can be confidently proposed.
The interface between emotion and cognition is fundamentally important in understanding mood disorder. We have described the clinical relevance of cognitive emotions such as guilt and shame and also the possible specificity of deficits in affective cognition to depression. In broad terms, depressed patients show biases toward negative material and/or away from positive material relative to controls. These biases cut across cognitive domains, being observed for recognition, attention, and memory. Neuroimaging studies suggest that these biases have an identifiable neuronal basis. Although the pattern of results is complex, there is general support for the view that disturbances of affective cognition are associated with imbalances in limbic-cortical circuitry. Phillips et al (2003, 2008) argued that emotional stimuli elicit heightened responses in limbic regions including the amygdala and that hypo-response in prefrontal regions reflects a failure of cortical systems to regulate emotional responses. Various iterations of Helen Mayberg's influential model emphasize enhanced amygdala response to emotional stimuli in the depressed state that normalizes on recovery. Critically, she places the subgenual ACC at the epicenter of the dysfunctional network, hypothesizing a critical role for this region in emotion regulation (Ressler and Mayberg, 2007) and demonstrates that in treatment-resistant patients, deep brain stimulation in this region causes dramatic symptom improvement (Mayberg et al, 2005; Mayberg, 2009). A central role for the subgenual ACC is interesting in the context of the moral emotion literature we have reviewed, suggesting that this region mediates self-blame, which is an important symptom of depression. Exciting recent research suggests that connectivity between the amygdala and ventral ACC, as well as the OFC, may be a critical substrate of disrupted affective cognition in depression.
We explored the possible role of 5-HT in modulating affective cognition and its disruption in depression. The results are complex and equivocal at times. Both behavioral and neuronal effects in controls are subtle and inconsistent, whereas in depressed patients, results are difficult to interpret in the context of effects of 5-HT manipulation on depressed mood. Overall, the evidence offers some support for serotonergic modulation of affective cognition. However, a number of intervening factors may be critical, including current mood, personality traits, gender, and genotype, all of which have been shown to interact with 5-HT effects. Task selection may also be crucial; eg, we reviewed 5-HT effects on long-term forgetting of emotional material rather than short-term recall, and effects on reaction times rather than response accuracy. Improved understanding of the complex relationships between 5-HT, mood, and affective cognition is critical for developing effective therapies for depression.
FUTURE RESEARCH DIRECTIONS
Developing Paradigms
Reviewing the literature on affective cognition, particularly in the context of 5-HT manipulation, highlights not only the gaps in our knowledge, but also the difficulty in interpreting and comparing studies. This is due to a lack of agreed and standardized tasks and, even when the same task is used, there is no specification of the primary outcome variable and a strong tendency to find and overinterpret secondary measures. It has long been agreed that this is not acceptable in clinical trials because of the danger of false positives, overestimates of effect size and bias, and we suggest that it is time to consider how this could be brought into the experimental field. Although novel tasks are vital for developing and testing specific neurocognitive hypotheses, this is not always helpful in a clinical context. Without standardization, it is difficult to make progress, replicate results, or identify gaps that need to be addressed. One solution would be to develop a standard set of tasks in both behavioral and imaging contexts with defined methodology, primary outcomes, and presentations, so that individual tasks could be incorporated into experimental designs alongside individual hypothesis-driven tasks and the data then made available, ideally on a common database for combining and shared analysis.
Characterizing Patients: Vulnerability and Remission
Just as there is a lack of standardization of tasks, there are also marked inconsistencies in patients recruited by different studies. This is true of currently depressed patients, who may have widely differing levels of severity and medication status, of remitted patients, who may have been remitted for different lengths of time and have different levels of ongoing treatment, and of premorbidly vulnerable individuals, who may be vulnerable on the basis of family history, personality, or genotype. Although it is essential to study all of these groups to obtain a complete picture, more systematic exploration of these differences is required and studies do not always provide (or have available) the information to make important cross-study comparisons. Again, it may be valuable to consider a standard shared set of measures, including genotyping, to include across studies and make available on a common database.
Connectivity Models of Normal and Disrupted Affective Cognition
At several points in this review, we have argued that interconnected network models provide the most plausible accounts of affective cognition. Different tasks may probe different components of such models. Several models, with clear commonalities, have been proposed and rapidly advancing modeling techniques within fMRI provide a means to explicitly test these. A range of techniques are available for exploring functional and effective connectivity between brain regions (Rogers et al, 2007). One technique currently being explored is DCM, which directly assesses functional integration across interconnected brain regions and offers a valuable method for testing connectivity hypotheses (see Daunizeau et al 2010 for a recent review). We argued that disrupted connectivity underpins disturbances of affective cognition in depression, and although DCM is a challenging technique in clinical contexts, new research suggests that it can be applied in a range of disorders providing valuable information to complement routine fMRI results (Rowe et al, 2010; Almeida et al, 2009; Schlosser et al, 2008, 2010; Goulden et al, 2010). DCM also offers the potential to explore modulatory effects of pharmacological interventions on connectivity (Goulden, 2010).
Biomarkers for Treatment Evaluation
We along with others (Harmer et al, 2010; Clark et al, 2009) have argued that disrupted affective cognition mediated by disrupted amygdala/medial prefrontal function may be an important biomarker for depression. This has important implications for treatment development. For example, Harmer et al (2010) have argued that emotional memory, assessed either behaviorally or (perhaps more robustly) by fMRI response, may represent a biomarker for effective antidepressant drug action. Nonpharmacological treatments that act on disrupted emotional circuitry are also being developed (Ressler and Mayberg, 2007), with the notable success of deep brain stimulation offering one exciting advance (Mayberg et al, 2005; Mayberg, 2009). Vagus nerve stimulation has also been explored as a potential approach to treatment resistant depression, and recent work suggests that this technique interferes with negative memory mediated by the ventral and medial PFC (Critchley et al, 2007). It is also important to note that recurrence of depression is rarely systematically explored and that further work is required to elucidate the changes in neurocognitive and serotonergic functions across depressive episodes with a view to developing more longitudinal treatment strategies aimed at relapse prevention (Robinson and Sahakian, 2008).
Another approach to treatment evaluation is to explore mechanisms of effective cognitive therapies. fMRI could be used to determine whether psychological therapies work through the neurocognitive processes they target, and whether in individuals, therapy has achieved the desired effect. In this context, developing our understanding of the neuronal basis of complex moral emotions in depression is critical, as these emotions are important targets of cognitive therapy. It has been shown that neuronal responses associated with affective cognition predict response to cognitive therapy (Fu et al, 2008a, 2008b; Costafreda et al, 2009) and exploring mechanisms of psychological treatments more directly is an obvious next step.
An ultimate aim of treatment development in depression would be to identify individuals at risk and intervene before major depression develops. Affective cognition offers a possible route toward this approach, given the evidence we have reviewed, suggesting that differences in affective cognition (both behaviorally and neuronally) may be detectable in at-risk individuals, eg, those with specific genotypes, a strong family history, significant life stress, or a combination of these factors. Establishing biomarkers of vulnerability offers the possibility of identifying people who may benefit from preemptive cognitive therapy to promote resilient patterns of affective cognition and prevent the onset of depression.
References
Abramson LY, Seligman ME, Teasdale JD (1978). Learned helplessness in humans: critique and reformulation. J Abnorm Psychol 87: 49–74. This seminal paper emphasizes global, stable and internal attributions for negative outcomes as key to understanding vulnerability to depression.
Adolphs R, Baron-Cohen S, Tranel D (2002). Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci 14: 1264–1274.
Adolphs R, Cahill L, Schul R, Babinsky R (1997). Impaired declarative memory for emotional material following bilateral amygdala damage in humans. Learn Mem 4: 291–300.
Adolphs R, Tranel D (2004). Impaired judgments of sadness but not happiness following bilateral amygdala damage. J Cogn Neurosci 16: 453–462.
Adolphs R, Tranel D, Damasio AR (1998). The human amygdala in social judgment. Nature 393: 470–474.
Adolphs R, Tranel D, Damasio H, Damasio A (1994). Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372: 669–672.
Adolphs R, Tranel D, Hamann S, Young AW, Calder AJ, Phelps EA et al (1999). Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia 37: 1111–1117.
Alexander B, Brewin CR, Vearnals S, Wolff G, Leff J (1999). An investigation of shame and guilt in a depressed sample. Br J Med Psychol 72: 323–338.
Almeida JR, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ et al (2009). Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry 66: 451–459. Important study showing how connectivity analysis can advance our understanding of the neuronal basis of mood disorder.
American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders – DSMIV-TR. American Psychiatric Association: Washington, DC.
Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L et al (2005). Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry 57: 1079–1088.
Anders S, Lotze M, Erb M, Grodd W, Birbaumer N (2004). Brain activity underlying emotional valence and arousal: a response-related fMRI study. Hum Brain Mapp 23: 200–209.
Anderson AK, Phelps EA (2000). Expression without recognition: contributions of the human amygdala to emotional communication. Psychol Sci 11: 106–111.
Anderson IM, Del-Ben CM, McKie S, Richardson P, Williams SR, Elliott R et al (2007). Citalopram modulation of neuronal responses to aversive face emotions: a functional MRI study. Neuroreport 18: 1351–1355. Neuroimaging study identifying effects of 5-HT agonism on face processing.
Anderson IM, McKie S, Elliott R, Williams SR, Deakin JF (2009). Assessing human 5-HT function in vivo with pharmacoMRI. Neuropharmacology 55: 1029–1037.
Andrews B (1995). Bodily shame as a mediator between abusive experiences and depression. J Abnorm Psychol 104: 277–285.
Arce E, Simmons AN, Lovero KL, Stein MB, Paulus MP (2008). Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacology (Berl) 196: 661–672.
Arnone D, Horder J, Cowen PJ, Harmer CJ (2009). Early effects of mirtazapine on emotional processing. Psychopharmacology (Berl) 203: 685–691.
Atchley RA, Ilardi SS, Enloe A (2003). Hemispheric asymmetry in the processing of emotional content in word meanings: the effect of current and past depression. Brain Lang 84: 105–119.
Attenburrow MJ, Williams C, Odontiadis J, Reed A, Powell J, Cowen PJ et al (2003). Acute administration of nutritionally sourced tryptophan increases fear recognition. Psychopharmacology (Berl) 169: 104–107.
Banos RM, Medina PM, Pascual J (2001). Explicit and implicit memory biases in depression and panic disorder. Behav Res Ther 39: 61–74.
Barry ES, Naus MJ, Rehm LP (2004). Depression and implicit memory: understanding mood congruent memory bias. Cogn Ther Res 28: 387–414.
Baumgartner T, Lutz K, Schmidt CF, Jancke L (2006). The emotional power of music: how music enhances the feeling of affective pictures. Brain Res 1075: 151–164.
Bazin N, Perruchet P, Feline A (1996). Mood congruence effect in explicit and implicit memory tasks: a comparison between depressed patients, schizophrenic patients and controls. Eur Psychiatry 11: 390–395.
Beauregard M, Leroux JM, Bergman S, Arzoumanian Y, Beaudoin G, Bourgouin P et al (1998). The functional neuroanatomy of major depression: an fMRI study using an emotional activation paradigm. Neuroreport 9: 3253–3258.
Bechara A, Damasio AR, Damasio H, Anderson SW (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50: 7–15.
Beck AT, Rush AJ, Shaw BF, Emery G (1979). Cognitive Therapy of Depression. Guilford Press: New York. This seminal book describes the cognitive therapy model of depression and highlights the role of depressiogenic cognitive distortions such as overgeneralization of negative experiences.
Beck AT (1967). Depression: Clinical, Experimental and Theoretical Aspects. Hoeber: New York.
Beer JS, John OP, Scabini D, Knight RT (2006). Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. J Cogn Neurosci 18: 871–879.
Beesdo K, Pine DS, Lieb R, Wittchen HU (2010). Incidence, risk patterns of anxiety, depressive disorders, categorization of generalized anxiety disorder. Arch Gen Psychiatry 67: 47–57.
Beevers CG, Ellis AJ, Wells TT, McGeary JE (2009a). Serotonin transporter gene promoter region polymorphism and selective processing of emotional images. Biol Psychol 83: 260–265.
Beevers CG, Gibb BE, McGeary JE, Miller IW (2007). Serotonin transporter genetic variation and biased attention for emotional word stimuli among psychiatric inpatients. J Abnorm Psychol 116: 208–212.
Beevers CG, Wells TT, Ellis AJ, McGeary JE (2009b). Association of the serotonin transporter gene promoter region (5-HTTLPR) polymorphism with biased attention for emotional stimuli. J Abnorm Psychol 118: 670–681.
Bermpohl F, Walter M, Sajonz B, Lucke C, Hagele C, Sterzer P et al (2009). Attentional modulation of emotional stimulus processing in patients with major depression–alterations in prefrontal cortical regions. Neurosci Lett 463: 108–113.
Berrios GE, Bulbena A, Bakshi N, Dening TR, Jenaway A, Markar H et al (1992). Feelings of guilt in major depression – conceptual and psychometric aspects. Br J Psychiatry 160: 781–787.
Berthoz S, Armony JL, Blair RJR, Dolan RJ (2002). An fMRI study of intentional and unintentional (embarrassing) violations of social norms. Brain 125: 1696–1708.
Bhagwagar Z, Cowen PJ (2008). It's not over when it's over': persistent neurobiological abnormalities in recovered depressed patients. Psychol Med 38: 307–313.
Bhagwagar Z, Cowen PJ, Goodwin GM, Harmer CJ (2004). Normalization of enhanced fear recognition by acute SSRI treatment in subjects with a previous history of depression. Am J Psychiatry 161: 166–168.
Bhagwagar Z, Hinz R, Taylor M, Fancy S, Cowen P, Grasby P (2006). Increased 5-HT(2A) receptor binding in euthymic, medication-free patients recovered from depression: a positron emission study with [(11)C]MDL 100,907. Am J Psychiatry 163: 1580–1587.
Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR (2008). Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology 33: 3221–3225. Shows that 5-HT manipulation directly affects amygdala function; read with Fischer et al (2006).
Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ (1999). Dissociable neural responses to facial expressions of sadness and anger. Brain 122 (Pt 5): 883–893.
Boucsein K, Weniger G, Mursch K, Steinhoff BJ, Irle E (2001). Amygdala lesion in temporal lobe epilepsy subjects impairs associative learning of emotional facial expressions. Neuropsychologia 39: 231–236.
Bouhuys AL, Geerts E, Gordijn MC (1999). Depressed patients' perceptions of facial emotions in depressed and remitted states are associated with relapse: a longitudinal study. J Nerv Ment Dis 187: 595–602.
Bozeat S, Gregory CA, Ralph MA, Hodges JR (2000). Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer's disease? J Neurol Neurosurg Psychiatry 69: 178–186.
Bradley BP, Mogg K, Millar N (1996). Implicit memory bias in clinical and non-clinical depression. Behav Res Ther 34: 865–879.
Bradley BP, Mogg K, Williams R (1995). Implicit and explicit memory for emotion-congruent information in clinical depression and anxiety. Behav Res Ther 33: 755–770.
Brassen S, Gamer M, Rose M, Buchel C (2010). The influence of directed covert attention on emotional face processing. Neuroimage 50: 545–551.
Brattico E, Jacobsen T (2009). Subjective appraisal of music: neuroimaging evidence. Ann NY Acad Sci 1169: 308–317.
Broomfield NM, Davies R, MacMahon K, Ali F, Cross SM (2007). Further evidence of attention bias for negative information in late life depression. Int J Geriatr Psychiatry 22: 175–180.
Brown GW, Bifulco A, Harris TO (1987). Life events, vulnerability and onset of depression: some refinements. Br J Psychiatry 150: 30–42.
Brown GW, Harris TO, Hepworth C (1995). Loss, humiliation and entrapment among women developing depression: a patient and non-patient comparison. Psychol Med 25: 7–21.
Brown SM, Peet E, Manuck SB, Williamson DE, Dahl RE, Ferrell RE et al (2005). A regulatory variant of the human tryptophan hydroxylase-2 gene biases amygdala reactivity. Mol Psychiatry 10: 884–888, 805.
Browning M, Reid C, Cowen PJ, Goodwin GM, Harmer CJ (2007). A single dose of citalopram increases fear recognition in healthy subjects. J Psychopharmacol 21: 684–690.
Buchanan TW, Denburg NL, Tranel D, Adolphs R (2001). Verbal and nonverbal emotional memory following unilateral amygdala damage. Learn Mem 8: 326–335.
Cahill L, McGaugh JL (1995). A novel demonstration of enhanced memory associated with emotional arousal. Conscious Cogn 4: 410–421.
Camille N, Coricelli G, Sallet J, Pradat-Diehl P, Duhamel JR, Sirigu A (2004). The involvement of the orbitofrontal cortex in the experience of regret. Science 304: 1167–1170.
Canli T, Amin Z, Haas B, Omura K, Constable RT (2004). A double dissociation between mood states and personality traits in the anterior cingulate. Behav Neurosci 118: 897–904.
Canli T, Congdon E, Gutknecht L, Constable RT, Lesch KP (2005). Amygdala responsiveness is modulated by tryptophan hydroxylase-2 gene variation. J Neural Transm 112: 1479–1485.
Canli T, Congdon E, Todd CR, Lesch KP (2008). Additive effects of serotonin transporter and tryptophan hydroxylase-2 gene variation on neural correlates of affective processing. Biol Psychol 79: 118–125.
Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L (2000). Event-related activation in the human amygdala associates with later memory for individual emotional experience. J Neurosci 20: RC99.
Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS et al (2007). Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C]DASB; comparison with bipolar disorder. Biol Psychiatry 62: 870–877.
Chamberlain SR, Sahakian BJ (2006). The neuropsychology of mood disorders. Curr Psychiatry Rep 8: 458–463.
Chan SW, Goodwin GM, Harmer CJ (2007). Highly neurotic never-depressed students have negative biases in information processing. Psychol Med 37: 1281–1291.
Chan SW, Harmer CJ, Goodwin GM, Norbury R (2008). Risk for depression is associated with neural biases in emotional categorisation. Neuropsychologia 46: 2896–2903.
Chan SW, Norbury R, Goodwin GM, Harmer CJ (2009). Risk for depression and neural responses to fearful facial expressions of emotion. Br J Psychiatry 194: 139–145. This paper presents evidence that never depressed individuals with vulnerability have altered neuronal processing of emotion.
Chen CH, Suckling J, Ooi C, Fu CH, Williams SC, Walsh ND et al (2008). Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology 33: 1909–1918.
Chiu PH, Holmes AJ, Pizzagalli DA (2008). Dissociable recruitment of rostral anterior cingulate and inferior frontal cortex in emotional response inhibition. Neuroimage 42: 988–997.
Ciaramelli E, Muccioli M, Ladavas E, di Pellegrino G (2007). Selective deficit in personal moral judgment following damage to ventromedial prefrontal cortex. Soc Cogn Affect Neurosci 2: 84–92.
Cicerone KD, Tanenbaum LN (1997). Disturbance of social cognition after traumatic orbitofrontal brain injury. Arch Clin Neuropsychol 12: 173–188.
Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW (2008). Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain 131: 1311–1322.
Clark L, Chamberlain SR, Sahakian BJ (2009). Neurocognitive mechanisms in depression: implications for treatment. Annu Rev Neurosci 32: 57–74.
Cools R, Calder AJ, Lawrence AD, Clark L, Bullmore E, Robbins TW (2005). Individual differences in threat sensitivity predict serotonergic modulation of amygdala response to fearful faces. Psychopharmacology (Berl) 180: 670–679.
Costafreda SG, Brammer MJ, David AS, Fu CH (2008). Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev 58: 57–70. Valuable and comprehensive meta-analysis on amygdala and emotional processing.
Costafreda SG, Khanna A, Mourao-Miranda J, Fu CH (2009). Neural correlates of sad faces predict clinical remission to cognitive behavioural therapy in depression. Neuroreport 20: 637–641.
Coupland NJ, Sustrik RA, Ting P, Li D, Hartfeil M, Singh AJ et al (2004). Positive and negative affect differentially influence identification of facial emotions. Depress Anxiety 19: 31–34.
Cowen PJ, Charig EM (1987). Neuroendocrine responses to intravenous tryptophan in major depression. Arch Gen Psychiatry 44: 958–966.
Cremers HR, Demenescu LR, Aleman A, Renken R, van Tol MJ, van der Wee NJ et al (2010). Neuroticism modulates amygdala-prefrontal connectivity in response to negative emotional facial expressions. Neuroimage 49: 963–970.
Critchley HD, Lewis PA, Orth M, Josephs O, Deichmann R, Trimble MR et al (2007). Vagus nerve stimulation for treatment-resistant depression: behavioral and neural effects on encoding negative material. Psychosom Med 69: 17–22.
Csukly G, Czobor P, Szily E, Takacs B, Simon L (2009). Facial expression recognition in depressed subjects: the impact of intensity level and arousal dimension. J Nerv Ment Dis 197: 98–103.
Damasio AR (1994). Descartes' Error: Emotion, Reason, and the Human Brain. Putnam Publishing, New York.
Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR (1994). The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science 264: 1102–1105. A seminal paper addressing the function of VMPFC.
Danion JM, Kauffmann-Muller F, Grange D, Zimmermann MA, Greth P (1995). Affective valence of words, explicit and implicit memory in clinical depression. J Affect Disord 34: 227–234.
Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, Heindel W et al (2007). Amygdala reactivity to masked negative faces is associated with automatic judgmental bias in major depression: a 3 T fMRI study. J Psychiatry Neurosci 32: 423–429.
Daunizeau J, David O, Stephan KE (2010). Dynamic causal modelling: a critical review of the biophysical and statistical foundations. Neuroimage (In press).
Davidson RJ, Irwin W, Anderle MJ, Kalin NH (2003). The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry 160: 64–75.
Deakin JF, Pennell I, Upadhyaya AJ, Lofthouse R (1990). A neuroendocrine study of 5HT function in depression: evidence for biological mechanisms of endogenous and psychosocial causation. Psychopharmacology (Berl) 101: 85–92.
Del-Ben CM, Deakin JF, McKie S, Delvai NA, Williams SR, Elliott R et al (2005). The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: an FMRI study. Neuropsychopharmacology 30: 1724–1734.
Delgado PL, Price LH, Miller HL, Salomon RM, Aghajanian GK, Heninger GR et al (1994). Serotonin and the neurobiology of depression. Effects of tryptophan depletion in drug-free depressed patients. Arch Gen Psychiatry 51: 865–874.
Demaree HA, Pu J, Jesberger J, Feeny N, Jeng L, Everhart DE et al (2009). 5HTTLPR predicts left fusiform gyrus activation to positive emotional stimuli. Magn Reson Imaging 27: 441–448.
Dichter GS, Felder JN, Smoski MJ (2009). Affective context interferes with cognitive control in unipolar depression: an fMRI investigation. J Affect Disord 114: 131–142.
Direnfeld DM, Roberts JE (2006). Mood congruent memory in dysphoria: the roles of state affect and cognitive style. Behav Res Ther 44: 1275–1285.
Dolan RJ (2002). Emotion, cognition, and behavior. Science 298: 1191–1194.
Dolan RJ (2007). The human amygdala and orbital prefrontal cortex in behavioural regulation. Philos Trans R Soc Lond B Biol Sci 362: 787–799.
Dolan RJ, Lane R, Chua P, Fletcher P (2000). Dissociable temporal lobe activations during emotional episodic memory retrieval. Neuroimage 11: 203–209.
Drevets WC, Savitz J (2008). The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums 13: 663–681. This review is an excellent summary of the more recent neuroimaging literature on the role of the subgenual cingulate in depression.
Dunbar GC, Lishman WA (1984). Depression, recognition-memory and hedonic tone a signal detection analysis. Br J Psychiatry 144: 376–382.
Ebert D, Ebmeier KP (1996). The role of the cingulate gyrus in depression: from functional anatomy to neurochemistry. Biol Psychiatry 39: 1044–1050.
Edith Frank J, Tomaz C (2003). Lateralized impairment of the emotional enhancement of verbal memory in patients with amygdala-hippocampus lesion. Brain Cogn 52: 223–230.
Edwards-Lee T, Miller B, Benson D, Cummings J, Russell G, Boone K et al (1997). The temporal variant of frontotemporal dementia. Brain 120: 1027–1040.
Eisenberg N (2000). Emotion, regulation, and moral development. Annu Rev Psychol 51: 665–697.
Ekman P, Friesen WV (1971). Constants across cultures in the face and emotion. J Pers Soc Psychol 17: 124–129.
Eldar E, Ganor O, Admon R, Bleich A, Hendler T (2007). Feeling the real world: limbic response to music depends on related content. Cereb Cortex 17: 2828–2840.
Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ (2000). Selective attention to emotional stimuli in a verbal Go/No-Go task: an fMRI study. Neuroreport 11: 1739–1744.
Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ (2002). The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry 59: 597–604. Clear demonstration that ventral ACC is a substrate for mood congruent attentional biases.
Ellwart T, Rinck M, Becker ES (2003). Selective memory and memory deficits in depressed inpatients. Depress Anxiety 17: 197–206.
Erickson K, Drevets WC, Clark L, Cannon DM, Bain EE, Zarate Jr CA et al (2005). Mood-congruent bias in affective Go/No-Go performance of unmedicated patients with major depressive disorder. Am J Psychiatry 162: 2171–2173.
Eslinger PJ, Damasio AR (1985). Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology 35: 1731–1741.
Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J (2006). Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51: 871–882.
Evers AT, der Veen MV, Jolles J, Deutz EP, Schmitt AJ (2009). The effect of acute tryptophan depletion on performance and the BOLD response during a Stroop task in healthy first-degree relatives of patients with unipolar depression. Psychiatry Res 173: 52–58.
Evers EA, van der Veen FM, Jolles J, Deutz NE, Schmitt JA (2006). Acute tryptophan depletion improves performance and modulates the BOLD response during a Stroop task in healthy females. Neuroimage 32: 248–255.
Fairhall SL, Ishai A (2007). Effective connectivity within the distributed cortical network for face perception. Cereb Cortex 17: 2400–2406. This study delineates and tests a distributed network for emotional face processing.
Fakra E, Hyde LW, Gorka A, Fisher PM, Munoz KE, Kimak M et al (2009). Effects of HTR1A C(-1019)G on amygdala reactivity and trait anxiety. Arch Gen Psychiatry 66: 33–40.
Fales CL, Barch DM, Rundle MM, Mintun MA, Mathews J, Snyder AZ et al (2009). Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord 112: 206–211.
Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD et al (2008). Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry 63: 377–384.
Farmer AE, McGuffin P (2003). Humiliation, loss and other types of life events and difficulties: a comparison of depressed subjects, healthy controls and their siblings. Psychol Med 33: 1169–1175.
Fava M, Hwang I, Rush AJ, Sampson N, Walters EE, Kessler RC (2010). The importance of irritability as a symptom of major depressive disorder: results from the National Comorbidity Survey Replication. Mol Psychiatry (In press).
Feinberg TE, Rifkin A, Schaffer C, Walker E (1986). Facial discrimination and emotional recognition in schizophrenia and affective disorders. Arch Gen Psychiatry 43: 276–279.
Fessler D (1999). Toward an understanding of the universality of second order emotions. In: Hinton A (ed). Beyond Nature or Nurture: Biocultural Approaches to the Emotions. Cambridge University Press: New York. pp 75–116.
Finlay-Jones R, Brown GW (1981). Types of stressful life event and the onset of anxiety and depressive disorders. Psychol Med 11: 803–815.
Firk C, Markus CR (2008). Effects of acute tryptophan depletion on affective processing in first-degree relatives of depressive patients and controls after exposure to uncontrollable stress. Psychopharmacology (Berl) 199: 151–160.
Fisher PM, Meltzer CC, Price JC, Coleman RL, Ziolko SK, Becker C et al (2009). Medial prefrontal cortex 5-HT(2A) density is correlated with amygdala reactivity, response habituation, and functional coupling. Cereb Cortex 19: 2499–2507.
Fisher PM, Meltzer CC, Ziolko SK, Price JC, Moses-Kolko EL, Berga SL et al (2006). Capacity for 5-HT1A-mediated autoregulation predicts amygdala reactivity. Nat Neurosci 9: 1362–1363. Study directly relating serotonin function to emotional response; read with Bigos et al (2008).
Fox E, Ridgewell A, Ashwin C (2009). Looking on the bright side: biased attention and the human serotonin transporter gene. Proc Biol Sci 276: 1747–1751.
Friedel E, Schlagenhauf F, Sterzer P, Park SQ, Bermpohl F, Strohle A et al (2009). 5-HTT genotype effect on prefrontal-amygdala coupling differs between major depression and controls. Psychopharmacology (Berl) 205: 261–271.
Frodl T, Bokde AL, Scheuerecker J, Lisiecka D, Schoepf V, Hampel H et al (2010). Functional connectivity bias of the orbitofrontal cortex in drug-free patients with major depression. Biol Psychiatry 67: 161–167.
Frokjaer VG, Mortensen EL, Nielsen FA, Haugbol S, Pinborg LH, Adams KH et al (2008). Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biol Psychiatry 63: 569–576.
Fu CH, Mourao-Miranda J, Costafreda SG, Khanna A, Marquand AF, Williams SC et al (2008a). Pattern classification of sad facial processing: toward the development of neurobiological markers in depression. Biol Psychiatry 63: 656–662.
Fu CH, Williams SC, Brammer MJ, Suckling J, Kim J, Cleare AJ et al (2007). Neural responses to happy facial expressions in major depression following antidepressant treatment. Am J Psychiatry 164: 599–607.
Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J et al (2004). Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry 61: 877–889. Excellent study showing normalisation of aberrant face processing in response to treatment.
Fu CH, Williams SC, Cleare AJ, Scott J, Mitterschiffthaler MT, Walsh ND et al (2008b). Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol Psychiatry 64: 505–512.
Furmark T, Henningsson S, Appel L, Ahs F, Linnman C, Pissiota A et al (2009). Genotype over-diagnosis in amygdala responsiveness: affective processing in social anxiety disorder. J Psychiatry Neurosci 34: 30–40.
Fusar-Poli P, Allen P, Lee F, Surguladze S, Tunstall N, Fu CH et al (2007). Modulation of neural response to happy and sad faces by acute tryptophan depletion. Psychopharmacology (Berl) 193: 31–44.
Gamer M, Buchel C (2009). Amygdala activation predicts gaze toward fearful eyes. J Neurosci 29: 9123–9126.
Garavan H, Pendergrass JC, Ross TJ, Stein EA, Risinger RC (2001). Amygdala response to both positively and negatively valenced stimuli. Neuroreport 12: 2779–2783.
Garrett AS, Maddock RJ (2006). Separating subjective emotion from the perception of emotion-inducing stimuli: an fMRI study. Neuroimage 33: 263–274.
Ge T, Kendrick KM, Feng J (2009). A novel extended Granger Causal model approach demonstrates brain hemispheric differences during face recognition learning. PLoS Comput Biol 5: e1000570.
Gemar MC, Segal ZV, Mayberg HS, Goldapple K, Carney C (2007). Changes in regional cerebral blood flow following mood challenge in drug-free, remitted patients with unipolar depression. Depress Anxiety 24: 597–601.
Ghatavi K, Nicolson R, MacDonald C, Osher S, Levitt A (2002). Defining guilt in depression: a comparison of subjects with major depression, chronic medical illness and healthy controls. J Affect Disord 68: 307–315.
Gibb BE, Benas JS, Grassia M, McGeary J (2009). Children's attentional biases and 5-HTTLPR genotype: potential mechanisms linking mother and child depression. J Clin Child Adolesc Psychol 38: 415–426.
Gilbert P, McEwan K, Bellew R, Mills A, Gale C (2009). The dark side of competition: how competitive behaviour and striving to avoid inferiority are linked to depression, anxiety, stress and self-harm. Psychol Psychother 82: 123–136.
Gilboa-Schechtman E, Erhard-Weiss D, Jeczemien P (2002). Interpersonal deficits meet cognitive biases: memory for facial expressions in depressed and anxious men and women. Psychiatry Res 113: 279–293.
Gorman JM (1996). Comorbid depression and anxiety spectrum disorders. Depress Anxiety 4: 160–168.
Gosselin N, Peretz I, Johnsen E, Adolphs R (2007). Amygdala damage impairs emotion recognition from music. Neuropsychologia 45: 236–244.
Gotlib IH, Kasch KL, Traill S, Joormann J, Arnow BA, Johnson SL (2004a). Coherence and specificity of information-processing biases in depression and social phobia. J Abnorm Psychol 113: 386–398.
Gotlib IH, Krasnoperova E, Yue DN, Joormann J (2004b). Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol 113: 121–135.
Gotlib IH, McCann CD (1984). Construct accessibility and depression: an examination of cognitive and affective factors. J Pers Soc Psychol 47: 427–439.
Gotlib IH, Sivers H, Gabrieli JD, Whitfield-Gabrieli S, Goldin P, Minor KL et al (2005). Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport 16: 1731–1734.
Goulden N, McKie S, Suckling J, Williams SR, Anderson IM, Deakin JF et al (2010). A comparison of permutation and parametric testing for between group effective connectivity differences using DCM. Neuroimage 50: 509–515.
Goulden N (2010). Connectivity Techniques in Psychiatry. University of Manchester. PhD Thesis, Manchester, UK.
Green AC, Baerentsen KB, Stodkilde-Jorgensen H, Wallentin M, Roepstorff A, Vuust P (2008). Music in minor activates limbic structures: a relationship with dissonance? Neuroreport 19: 711–715.
Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD (2004). The neural bases of cognitive conflict and control in moral judgment. Neuron 44: 389–400.
Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD (2001). An fMRI investigation of emotional engagement in moral judgment. Science 293: 2105–2108.
Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC (1992). Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Res 42: 241–251.
Haas BW, Omura K, Amin Z, Constable RT, Canli T (2006). Functional connectivity with the anterior cingulate is associated with extraversion during the emotional Stroop task. Soc Neurosci 1: 16–24.
Hale III WW (1998). Judgment of facial expressions and depression persistence. Psychiatry Res 80: 265–274.
Hale III WW, Jansen JH, Bouhuys AL, van den Hoofdakker RH (1998). The judgement of facial expressions by depressed patients, their partners and controls. J Affect Disord 47: 63–70.
Hamann SB, Cahill L, McGaugh JL, Squire LR (1997). Intact enhancement of declarative memory for emotional material in amnesia. Learn Mem 4: 301–309.
Hamilton JP, Gotlib IH (2008). Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychiatry 63: 1155–1162. This study identifies a neuronal basis for negative memory biases in depression.
Harbaugh WT, Mayr U, Burghart DR (2007). Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science 316: 1622–1625.
Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ (2005). Contributions of amygdala and striatal activity in emotion regulation. Biol Psychiatry 57: 624–632.
Harmer CJ, Cowen PJ, Goodwin GM (2010). Efficacy markers in depression. J Psychopharmacol (In press).
Harmer CJ, Bhagwagar Z, Perrett DI, Vollm BA, Cowen PJ, Goodwin GM (2003a). Acute SSRI administration affects the processing of social cues in healthy volunteers. Neuropsychopharmacology 28: 148–152.
Harmer CJ, Heinzen J, O'Sullivan U, Ayres RA, Cowen PJ (2008). Dissociable effects of acute antidepressant drug administration on subjective and emotional processing measures in healthy volunteers. Psychopharmacology (Berl) 199: 495–502.
Harmer CJ, Hill SA, Taylor MJ, Cowen PJ, Goodwin GM (2003b). Toward a neuropsychological theory of antidepressant drug action: increase in positive emotional bias after potentiation of norepinephrine activity. Am J Psychiatry 160: 990–992.
Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM (2006a). Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry 59: 816–820.
Harmer CJ, O'Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A et al (2009). Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry 166: 1178–1184. This study addresses the state-trait issue and shows that negative biases respond acutely to antidepressants, prior to mood effects.
Harmer CJ, Reid CB, Ray MK, Goodwin GM, Cowen PJ (2006b). 5HT(3) antagonism abolishes the emotion potentiated startle effect in humans. Psychopharmacology (Berl) 186: 18–24.
Harmer CJ, Rogers RD, Tunbridge E, Cowen PJ, Goodwin GM (2003c). Tryptophan depletion decreases the recognition of fear in female volunteers. Psychopharmacology (Berl) 167: 411–417.
Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM (2004). Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry 161: 1256–1263. Study exploring differential neurotransmitter effects on face processing.
Haxby JV, Hoffman EA, Gobbini MI (2002). Human neural systems for face recognition and social communication. Biol Psychiatry 51: 59–67.
Hayward G, Goodwin GM, Cowen PJ, Harmer CJ (2005). Low-dose tryptophan depletion in recovered depressed patients induces changes in cognitive processing without depressive symptoms. Biol Psychiatry 57: 517–524.
Hedlund S, Rude SS (1995). Evidence of latent depressive schemas in formerly depressed individuals. J Abnorm Psychol 104: 517–525.
Heekeren HR, Wartenburger I, Schmidt H, Schwintowski HP, Villringer A (2003). An fMRI study of simple ethical decision-making. Neuroreport 14: 1215–1219.
Hill AB, Knowles TH (1991). Depression and the ‘emotional’ Stroop effect. Pers Individ Diff 12: 481–485.
Hill J, Pickles A, Burnside E, Byatt M, Rollinson L, Davis R et al (2001). Child sexual abuse, poor parental care and adult depression: evidence for different mechanisms. Br J Psychiatry 179: 104–109.
Hood SD, Bell CJ, Nutt DJ (2005). Acute tryptophan depletion. Part I: rationale and methodology. Aust NZJ Psychiatry 39: 558–564.
Hsu M, Anen C, Quartz SR (2008). The right and the good: distributive justice and neural encoding of equity and efficiency. Science 320: 1092–1095.
Hughes JM, Matrenza C, Kemp AH, Harrison BJ, Liley D, Nathan PJ (2004). Selective effects of simultaneous monoamine depletion on mood and emotional responsiveness. Int J Neuropsychopharmacol 7: 9–17.
Ishai A, Pessoa L, Bikle PC, Ungerleider LG (2004). Repetition suppression of faces is modulated by emotion. Proc Natl Acad Sci USA 101: 9827–9832.
Janoff-Bulman R (1979). Characterological versus behavioral self-blame – inquiries into depression and rape. J Pers Soc Psychol 37: 1798–1809.
Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ (2007). Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci 27: 8877–8884.
Joormann J, Gotlib IH (2006). Is this happiness I see? Biases in the identification of emotional facial expressions in depression and social phobia. J Abnorm Psychol 115: 705–714.
Joormann J, Gotlib IH (2007). Selective attention to emotional faces following recovery from depression. J Abnorm Psychol 116: 80–85.
Juhasz G, Chase D, Pegg E, Downey D, Toth ZG, Stones K et al (2009). CNR1 gene is associated with high neuroticism and low agreeableness and interacts with recent negative life events to predict current depressive symptoms. Neuropsychopharmacology 34: 2019–2027.
Kapitany T, Schindl M, Schindler SD, Hesselmann B, Fureder T, Barnas C et al (1999). The citalopram challenge test in patients with major depression and in healthy controls. Psychiatry Res 88: 75–88.
Kedia G, Berthoz S, Wessa M, Hilton D, Martinot JL (2008). An agent harms a victim: a functional magnetic resonance imaging study on specific moral emotions. J Cogn Neurosci 20: 1788–1798.
Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML (2005). A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol Psychiatry 58: 495–503.
Kendler KS, Kuhn J, Prescott CA (2004). The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am J Psychiatry 161: 631–636.
Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ (1992). Major depression and generalized anxiety disorder. Same genes (partly) different environments? Arch Gen Psychiatry 49: 716–722.
Kensinger EA, Schacter DL (2006). Processing emotional pictures and words: effects of valence and arousal. Cogn Affect Behav Neurosci 6: 110–126.
Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR et al (2003). The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289: 3095–3105.
Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ (2003). Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport 14: 2317–2322.
Klaassen T, Riedel WJ, Deutz NE, van Praag HM (2002). Mood congruent memory bias induced by tryptophan depletion. Psychol Med 32: 167–172. Shows that effects of ATD on memory may critically depend on delay of recall.
Koelsch S, Fritz T, DY VC, Muller K, Friederici AD (2006). Investigating emotion with music: an fMRI study. Hum Brain Mapp 27: 239–250.
Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M et al (2007). Damage to the prefrontal cortex increases utilitarian moral judgements. Nature 446: 908–911.
Krueger F, McCabe K, Moll J, Kriegeskorte N, Zahn R, Strenziok M et al (2007). Neural correlates of trust. Proc Natl Acad Sci USA 104: 20084–20089.
Kuchinke L, Jacobs AM, Vo ML, Conrad M, Grubich C, Herrmann M (2006). Modulation of prefrontal cortex activation by emotional words in recognition memory. Neuroreport 17: 1037–1041.
Kumari V, Mitterschiffthaler MT, Teasdale JD, Malhi GS, Brown RG, Giampietro V et al (2003). Neural abnormalities during cognitive generation of affect in treatment-resistant depression. Biol Psychiatry 54: 777–791.
Kyte ZA, Goodyer IM, Sahakian BJ (2005). Selected executive skills in adolescents with recent first episode major depression. J Child Psychol Psychiatry 46: 995–1005.
Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ et al (1997). Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia 35: 1437–1444.
Lang PJ, Bradley MM, Cuthbert BN (2008). International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Technical Report A-8. University of Florida: Gainesville, FL.
Lau JY, Goldman D, Buzas B, Fromm SJ, Guyer AE, Hodgkinson C et al (2009). Amygdala function and 5-HTT gene variants in adolescent anxiety and major depressive disorder. Biol Psychiatry 65: 349–355.
Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C et al (2004). Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry 55: 578–587.
Le Masurier M, Cowen PJ, Harmer CJ (2007). Emotional bias and waking salivary cortisol in relatives of patients with major depression. Psychol Med 37: 403–410.
Leckman JF, Caruso KA, Prusoff BA, Weissman MM, Merikangas KR, Pauls DL (1984). Appetite disturbance and excessive guilt in major depression – use of family study data to define depressive subtypes. Arch Gen Psychiatry 41: 839–844.
Lee BT, Lee HY, Lee BC, Pae CU, Yoon BJ, Ryu SG et al (2009). Impact of the tryptophan hydroxylase 1 gene A218C polymorphism on amygdala activity in response to affective facial stimuli in patients with major depressive disorder. Genes Brain Behav 8: 512–518.
Lee BT, Seok JH, Lee BC, Cho SW, Yoon BJ, Lee KU et al (2008). Neural correlates of affective processing in response to sad and angry facial stimuli in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 32: 778–785.
Lemogne C, Bergouignan L, Boni C, Gorwood P, Pelissolo A, Fossati P (2009). Genetics and personality affect visual perspective in autobiographical memory. Conscious Cogn 18: 823–830.
Lemogne C, Piolino P, Friszer S, Claret A, Girault N, Jouvent R et al (2006). Episodic autobiographical memory in depression: specificity, autonoetic consciousness, and self-perspective. Conscious Cogn 15: 258–268.
Leppanen JM (2006). Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry 19: 34–39.
Leppanen JM, Milders M, Bell JS, Terriere E, Hietanen JK (2004). Depression biases the recognition of emotionally neutral faces. Psychiatry Res 128: 123–133.
Lewis PA, Critchley HD, Rotshtein P, Dolan RJ (2007). Neural correlates of processing valence and arousal in affective words. Cereb Cortex 17: 742–748.
Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM (2007). Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci 18: 421–428. Indicates the importance of cognitive demands on affective brain responses.
Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT (2000). Differential limbic-cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol Psychiatry 48: 30–42.
Liu W, Miller BL, Kramer JH, Rankin K, Wyss-Coray C, Gearhart R et al (2004). Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology 62: 742–748.
Mah L, Arnold MC, Grafman J (2004). Impairment of social perception associated with lesions of the prefrontal cortex. Am J Psychiatry 161: 1247–1255.
Mannie ZN, Bristow GC, Harmer CJ, Cowen PJ (2007). Impaired emotional categorisation in young people at increased familial risk of depression. Neuropsychologia 45: 2975–2980.
Markowitsch HJ (1998). Differential contribution of right and left amygdala to affective information processing. Behav Neurol 11: 233–244.
Markowitsch HJ, Calabrese P, Wurker M, Durwen HF, Kessler J, Babinsky R et al (1994). The amygdala's contribution to memory—a study on two patients with Urbach-Wiethe disease. Neuroreport 5: 1349–1352.
Marsh AA, Finger EC, Buzas B, Soliman N, Richell RA, Vythilingham M et al (2006). Impaired recognition of fear facial expressions in 5-HTTLPR S-polymorphism carriers following tryptophan depletion. Psychopharmacology (Berl) 189: 387–394.
Mathews A, Ridgeway V, Williamson DA (1996). Evidence for attention to threatening stimuli in depression. Behav Res Ther 34: 695–705.
Matthews SC, Strigo IA, Simmons AN, Yang TT, Paulus MP (2008). Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J Affect Disord 111: 13–20.
Mayberg HS (2009). Targeted electrode-based modulation of neural circuits for depression. J Clin Invest 119: 717–725.
Mayberg HS (1997). Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci 9: 471–481.
Mayberg HS (2002). Modulating limbic-cortical circuits in depression: targets of antidepressant treatments. Semin Clin Neuropsychiatry 7: 255–268.
Mayberg HS (2003). Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull 65: 193–207.
Mayberg HS (2007). Defining the neural circuitry of depression: toward a new nosology with therapeutic implications. Biol Psychiatry 61: 729–730. One of a series of studies refining a neural circuitry model for depression and relating this to treatment response.
Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA et al (1999). Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 156: 675–682.
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C et al (2005). Deep brain stimulation for treatment-resistant depression. Neuron 45: 651–660. This seminal study shows that direct stimulation of ACC dramatically improves depressed mood.
McCabe SB, Gotlib IH, Martin RA (2000). Cognitive vulnerability to depression: deployment-of-attention as a function of history of depression and current mood state. Cogn Ther Res 24: 427–444.
Mendelsohn D, Riedel WJ, Sambeth A (2009). Effects of acute tryptophan depletion on memory, attention and executive functions: a systematic review. Neurosci Biobehav Rev 33: 926–952.
Mendez MF, Anderson E, Shapira JS (2005). An investigation of moral judgement in frontotemporal dementia. Cogn Behav Neurol 18: 193–197.
Merens W, Booij L, Haffmans PJ, van der DA (2008). The effects of experimentally lowered serotonin function on emotional information processing and memory in remitted depressed patients. J Psychopharmacol 22: 653–662.
Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, Garcia A et al (2006). Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry 63: 1209–1216.
Meyer JH, Houle S, Sagrati S, Carella A, Hussey DF, Ginovart N et al (2004). Brain serotonin transporter binding potential measured with carbon 11-labeled DASB positron emission tomography: effects of major depressive episodes and severity of dysfunctional attitudes. Arch Gen Psychiatry 61: 1271–1279.
Meyer JH, McMain S, Kennedy SH, Korman L, Brown GM, DaSilva JN et al (2003). Dysfunctional attitudes and 5-HT2 receptors during depression and self-harm. Am J Psychiatry 160: 90–99.
Mickley Steinmetz KR, Kensinger EA (2009). The effects of valence and arousal on the neural activity leading to subsequent memory. Psychophysiology 46: 1190–1199.
Mikhailova ES, Vladimirova TV, Iznak AF, Tsusulkovskaya EJ, Sushko NV (1996). Abnormal recognition of facial expression of emotions in depressed patients with major depression disorder and schizotypal personality disorder. Biol Psychiatry 40: 697–705.
Mitterschiffthaler MT, Fu CH, Dalton JA, Andrew CM, Williams SC (2007). A functional MRI study of happy and sad affective states induced by classical music. Hum Brain Mapp 28: 1150–1162.
Mitterschiffthaler MT, Kumari V, Malhi GS, Brown RG, Giampietro VP, Brammer MJ et al (2003). Neural response to pleasant stimuli in anhedonia: an fMRI study. Neuroreport 14: 177–182.
Mogg K, Baldwin DS, Brodrick P, Bradley BP (2004). Effect of short-term SSRI treatment on cognitive bias in generalised anxiety disorder. Psychopharmacology (Berl) 176: 466–470.
Mogg K, Bradley BP, Williams R (1995). Attentional bias in anxiety and depression: the role of awareness. Br J Clin Psychol 34 (Pt 1): 17–36.
Moll J, de Oliveira-Souza R, Bramati IE, Grafman J (2002). Functional networks in emotional moral and nonmoral social judgments. Neuroimage 16: 696–703.
Moll J, de Oliveira-Souza R, Garrido GJ, Bramati IE, Caparelli-Daquer EMA, Paiva MMF et al (2007). The self as a moral agent: linking the neural bases of social agency and moral sensitivity. Soc Neurosci 2: 336–352.
Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J (2006). Human fronto-mesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci USA 103: 15623–15628.
Moll J, Schulkin J (2009). Social attachment and aversion in human moral cognition. Neurosci Biobehav Rev 33: 456–465. This review links recent work on the neurobiology and neurochemistry of attachment with insights into the neuroanatomical basis of moral emotions which critically depend on social attachment mechanisms.
Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J (2005). Opinion: the neural basis of human moral cognition. Nat Rev Neurosci 6: 799–809.
Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton III JL et al (2008). Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry 165: 90–98.
Moretti L, Dragone D, di Pellegrino G (2009). Reward and social valuation deficits following ventromedial prefrontal damage. J Cogn Neurosci 21: 128–140.
Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ et al (1998). A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain 121 (Pt 1): 47–57.
Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ et al (1996). A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 383: 812–815.
Munafo MR, Brown SM, Hariri AR (2008). Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry 63: 852–857. Comprehensive meta-analysis of this complex area.
Munafo MR, Hayward G, Harmer C (2006). Selective processing of social threat cues following acute tryptophan depletion. J Psychopharmacol 20: 33–39.
Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW et al (1999). Emotional bias and inhibitory control processes in mania and depression. Psychol Med 29: 1307–1321.
Murphy FC, Smith KA, Cowen PJ, Robbins TW, Sahakian BJ (2002). The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacology (Berl) 163: 42–53.
Murphy SE, Longhitano C, Ayres RE, Cowen PJ, Harmer CJ (2006). Tryptophan supplementation induces a positive bias in the processing of emotional material in healthy female volunteers. Psychopharmacology (Berl) 187: 121–130.
Murphy SE, Norbury R, O'Sullivan U, Cowen PJ, Harmer CJ (2009a). Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry 194: 535–540.
Murphy SE, Yiend J, Lester KJ, Cowen PJ, Harmer CJ (2009b). Short-term serotonergic but not noradrenergic antidepressant administration reduces attentional vigilance to threat in healthy volunteers. Int J Neuropsychopharmacol 12: 169–179.
Narabayashi H, Nagao T, Saito Y, Yoshida M, Nagahata M (1963). Stereotaxic amygdalotomy for behavior disorders. Arch Neurol 9: 1–16.
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S et al (1998). Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51: 1546–1554.
Neshat-Doost HT, Moradi AR, Taghavi MR, Yule W, Dalgleish T (2000). Lack of attentional bias for emotional information in clinically depressed children and adolescents on the dot probe task. J Child Psychol Psychiatry 41: 363–368.
Neumeister A, Drevets WC, Belfer I, Luckenbaugh DA, Henry S, Bonne O et al (2006). Effects of an alpha 2C-adrenoreceptor gene polymorphism on neural responses to facial expressions in depression. Neuropsychopharmacology 31: 1750–1756.
Nielen MM, Heslenfeld DJ, Heinen K, Van Strien JW, Witter MP, Jonker C et al (2009). Distinct brain systems underlie the processing of valence and arousal of affective pictures. Brain Cogn 71: 387–396.
Nolen-Hoeksema S (2000). The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol 109: 504–511.
Norbury R, Taylor MJ, Selvaraj S, Murphy SE, Harmer CJ, Cowen PJ (2009). Short-term antidepressant treatment modulates amygdala response to happy faces. Psychopharmacology (Berl) 206: 197–204.
Ochsner KN, Gross JJ (2005). The cognitive control of emotion. Trends Cogn Sci 9: 242–249.
Ochsner KN, Gross JJ (2007). The neural architecture of emotion regulation. In: Gross JJ and Buck R (eds). The Handbook of Emotion Regulation. Guilford Press: New York. pp 87–109.
O'Connor LE, Berry JW, Weiss J, Bush M, Sampson H (1997). Interpersonal guilt: the development of a new measure. J Clin Psychol 53: 73–89.
O'Connor LE, Berry JW, Weiss J, Gilbert P (2002). Guilt, fear, submission, and empathy in depression. J Affect Disord 71: 19–27. This paper shows the importance of exaggerated interpersonal guilt in people with major depression.
Osinsky R, Reuter M, Kupper Y, Schmitz A, Kozyra E, Alexander N et al (2008). Variation in the serotonin transporter gene modulates selective attention to threat. Emotion 8: 584–588.
Osinsky R, Schmitz A, Alexander N, Kuepper Y, Kozyra E, Hennig J (2009). TPH2 gene variation and conflict processing in a cognitive and an emotional Stroop task. Behav Brain Res 198: 404–410.
Paradiso S, Robinson RG, Andreasen NC, Downhill JE, Davidson RJ, Kirchner PT et al (1997). Emotional activation of limbic circuitry in elderly normal subjects in a PET study. Am J Psychiatry 154: 384–389.
Parsey RV, Hastings RS, Oquendo MA, Hu X, Goldman D, Huang YY et al (2006). Effect of a triallelic functional polymorphism of the serotonin-transporter-linked promoter region on expression of serotonin transporter in the human brain. Am J Psychiatry 163: 48–51.
Persad SM, Polivy J (1993). Differences between depressed and nondepressed individuals in the recognition of and response to facial emotional cues. J Abnorm Psychol 102: 358–368.
Pessoa L (2008). On the relationship between emotion and cognition. Nat Rev Neurosci 9: 148–158.
Pessoa L, Padmala S, Morland T (2005). Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. Neuroimage 28: 249–255.
Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS et al (2005). 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci 8: 828–834. Shows that genotype modulates crucial connectivity of amygdala.
Phan KL, Wager T, Taylor SF, Liberzon I (2002). Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348.
Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D (2009). Damage to association fiber tracts impairs recognition of the facial expression of emotion. J Neurosci 29: 15089–15099.
Phillips ML, Drevets WC, Rauch SL, Lane R (2003). Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry 54: 515–528. This paper proposes how a model of emotion perception may help understand mood disorders.
Phillips ML, Ladouceur CD, Drevets WC (2008). A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 13: 829, 833–857.
Phillips ML, Williams LM, Heining M, Herba CM, Russell T, Andrew C et al (2004). Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. Neuroimage 21: 1484–1496.
Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ et al (1997). A specific neural substrate for perceiving facial expressions of disgust. Nature 389: 495–498.
Posamentier MT, Abdi H (2003). Processing faces and facial expressions. Neuropsychol Rev 13: 113–143.
Price LH, Charney DS, Delgado PL, Heninger GR (1991). Serotonin function and depression: neuroendocrine and mood responses to intravenous L-tryptophan in depressed patients and healthy comparison subjects. Am J Psychiatry 148: 1518–1525.
Prosen M, Clark DC, Harrow M, Fawcett J (1983). Guilt and conscience in major depressive-disorders. Am J Psychiatry 140: 839–844.
Ramel W, Goldin PR, Eyler LT, Brown GG, Gotlib IH, McQuaid JR (2007). Amygdala reactivity and mood-congruent memory in individuals at risk for depressive relapse. Biol Psychiatry 61: 231–239.
Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW et al (2006). Structural anatomy of empathy in neurodegenerative disease. Brain 129: 2945–2956.
Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ et al (1997). Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry 154: 918–925.
Ressler KJ, Mayberg HS (2007). Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 10: 1116–1124.
Rhodes RA, Murthy NV, Dresner MA, Selvaraj S, Stavrakakis N, Babar S et al (2007). Human 5-HT transporter availability predicts amygdala reactivity in vivo. J Neurosci 27: 9233–9237.
Richell RA, Anderson IM (2001). Effects of tryptophan depletion on the recall of emotional material in healthy subjects. J Psychopharmacol 15: A13.
Rinck M, Becker ES (2005). A comparison of attentional biases and memory biases in women with social phobia and major depression. J Abnorm Psychol 114: 62–74.
Roberson-Nay R, McClure EB, Monk CS, Nelson EE, Guyer AE, Fromm SJ et al (2006). Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: an FMRI study. Biol Psychiatry 60: 966–973.
Robinson OJ, Sahakian BJ (2008). Recurrence in major depressive disorder: a neurocognitive perspective. Psychol Med 38: 315–318.
Robinson OJ, Sahakian BJ (2009). A double dissociation in the roles of serotonin and mood in healthy subjects. Biol Psychiatry 65: 89–92.
Rogers BP, Morgan VL, Newton AT, Gore JC (2007). Assessing functional connectivity in the human brain by fMRI. Magn Reson Imaging 25: 1347–1357.
Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K et al (1999). Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology 20: 322–339.
Roiser JP, Levy J, Fromm SJ, Nugent AC, Talagala SL, Hasler G et al (2009). The effects of tryptophan depletion on neural responses to emotional words in remitted depression. Biol Psychiatry 66: 441–450. Shows that ATD differentially affects ACC response to emotional stimuli in remitted patients.
Roiser JP, Levy J, Fromm SJ, Wang H, Hasler G, Sahakian BJ et al (2008). The effect of acute tryptophan depletion on the neural correlates of emotional processing in healthy volunteers. Neuropsychopharmacology 33: 1992–2006.
Roiser JP, Muller U, Clark L, Sahakian BJ (2007). The effects of acute tryptophan depletion and serotonin transporter polymorphism on emotional processing in memory and attention. Int J Neuropsychopharmacol 10: 449–461.
Rowe JB, Hughes LE, Barker RA, Owen AM (2010). Dynamic causal modelling of effective connectivity from fMRI: are results reproducible and sensitive to Parkinson's disease and its treatment? Neuroimage (In press).
Rubinow DR, Post RM (1992). Impaired recognition of affect in facial expression in depressed patients. Biol Psychiatry 31: 947–953.
Rubinsztein JS, Rogers RD, Riedel WJ, Mehta MA, Robbins TW, Sahakian BJ (2001). Acute dietary tryptophan depletion impairs maintenance of ‘affective set’ and delayed visual recognition in healthy volunteers. Psychopharmacology (Berl) 154: 319–326.
Ruhe HG, Mason NS, Schene AH (2007). Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry 12: 331–359. Important meta-analysis of depletion studies and mood.
Ruiz-Caballero JA, Bermudez J (1995). Neuroticism, mood, and retrieval of negative personal memories. J Gen Psychol 122: 29–35.
Rusch N, Lieb K, Gottler I, Hermann C, Schramm E, Richter H et al (2007). Shame and implicit self-concept in women with borderline personality disorder. Am J Psychiatry 164: 500–508.
Sartorius N, Jablensky A, Gulbinat W, Ernberg G (1980). WHO collaborative study: assessment of depressive disorders. Psychol Med 10: 743–749. This large transcultural study of depressive disorders shows that symptoms of feelings of worthlessness and guilt consistently occur across cultures, but that the latter is confined to only a certain proportion of patients.
Satterthwaite TD, Wolf DH, Gur RC, Ruparel K, Valdez JN, Gur RE et al (2009). Frontolimbic responses to emotional face memory: the neural correlates of first impressions. Hum Brain Mapp 30: 3748–3758.
Schaefer HS, Putnam KM, Benca RM, Davidson RJ (2006). Event-related functional magnetic resonance imaging measures of neural activity to positive social stimuli in pre- and post-treatment depression. Biol Psychiatry 60: 974–986.
Schlosser RG, Wagner G, Koch K, Dahnke R, Reichenbach JR, Sauer H (2008). Fronto-cingulate effective connectivity in major depression: a study with fMRI and dynamic causal modeling. Neuroimage 43: 645–655.
Schlosser RG, Wagner G, Schachtzabel C, Peikert G, Koch K, Reichenbach JR et al (2010). Fronto-cingulate effective connectivity in obsessive compulsive disorder: a study with fMRI, dynamic causal modeling. Hum Brain Mapp (In press).
Schulz KP, Clerkin SM, Halperin JM, Newcorn JH, Tang CY, Fan J (2009). Dissociable neural effects of stimulus valence and preceding context during the inhibition of responses to emotional faces. Hum Brain Mapp 30: 2821–2833.
Segal ZV, Gemar M, Truchon C, Guirguis M, Horowitz LM (1995). A priming methodology for studying self-representation in major depressive disorder. J Abnorm Psychol 104: 205–213.
Selvaraj S, Venkatesha Murthy N, Bhagwagar Z, Bose SK, Hinz R, Grasby PM et al (2010). Diminished brain 5-HT transporter binding in major depression: a positron emission tomography study with [(11)C]DASB. Psychopharmacology (Berl) (In press).
Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z et al (2004). Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage 22: 409–418.
Sergerie K, Chochol C, Armony JL (2008). The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 32: 811–830.
Sergerie K, Lepage M, Armony JL (2007). Influence of emotional expression on memory recognition bias: a functional magnetic resonance imaging study. Biol Psychiatry 62: 1126–1133.
Serretti A, Kato M, De Ronchi D, Kinoshita T (2007). Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry 12: 247–257.
Shamay-Tsoory SG, Aharon-Peretz J (2007). Dissociable prefrontal networks for cognitive and affective theory of mind: a lesion study. Neuropsychologia 45: 3054–3067.
Shamay-Tsoory SG, Aharon-Peretz J, Perry D (2009). Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 132: 617–627.
Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA (2001). Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry 50: 651–658.
Shin LM, Dougherty DD, Orr SP, Pitman RK, Lasko M, Macklin ML et al (2000). Activation of anterior paralimbic structures during guilt-related script-driven imagery. Biol Psychiatry 48: 43–50. This is the first functional imaging study on the neuroanatomical correlates of guilt and shows activation of the anterior cingulate dorsally to the genu of the corpus callosum.
Siegle GJ, Carter CS, Thase ME (2006). Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry 163: 735–738.
Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS (2002). Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry 51: 693–707.
Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME (2007). Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry 61: 198–209. An important study exploring the relationship between amygdala and lateral frontal function in depression.
Silvert L, Lepsien J, Fragopanagos N, Goolsby B, Kiss M, Taylor JG et al (2007). Influence of attentional demands on the processing of emotional facial expressions in the amygdala. Neuroimage 38: 357–366.
Smith AP, Henson RN, Dolan RJ, Rugg MD (2004). fMRI correlates of the episodic retrieval of emotional contexts. Neuroimage 22: 868–878.
Smith AP, Stephan KE, Rugg MD, Dolan RJ (2006). Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron 49: 631–638. Clear demonstration that the brain basis of affective cognition critically depends on task demands.
Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H (1998). Neural structures associated with recognition of facial expressions of basic emotions. Proc Biol Sci 265: 1927–1931.
Stark R, Zimmermann M, Kagerer S, Schienle A, Walter B, Weygandt M et al (2007). Hemodynamic brain correlates of disgust and fear ratings. Neuroimage 37: 663–673.
Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS et al (2007). A validated network of effective amygdala connectivity. Neuroimage 36: 736–745.
Stone VE, Baron-Cohen S, Calder A, Keane J, Young A (2003). Acquired theory of mind impairments in individuals with bilateral amygdala lesions. Neuropsychologia 41: 209–220.
Strange BA, Dolan RJ (2006). Anterior medial temporal lobe in human cognition: memory for fear and the unexpected. Cogn Neuropsychiatry 11: 198–218.
Strange BA, Kroes MC, Roiser JP, Tan GC, Dolan RJ (2008). Emotion-induced retrograde amnesia is determined by a 5-HTT genetic polymorphism. J Neurosci 28: 7036–7039.
Stuss DT, Benson DF, Clermont R, Della Malva CL, Kaplan EF, Weir WS (1986). Language functioning after bilateral prefrontal leukotomy. Brain Lang 28: 66–70.
Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ et al (2005). A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry 57: 201–209.
Surguladze SA, Elkin A, Ecker C, Kalidindi S, Corsico A, Giampietro V et al (2008). Genetic variation in the serotonin transporter modulates neural system-wide response to fearful faces. Genes Brain Behav 7: 543–551.
Surguladze SA, Young AW, Senior C, Brebion G, Travis MJ, Phillips ML (2004). Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology 18: 212–218.
Takahashi H, Yahata N, Koeda M, Matsuda T, Asai K, Okubo Y (2004). Brain activation associated with evaluative processes of guilt and embarrassment: an fMRI study. Neuroimage 23: 967–974.
Takahashi H, Yahata N, Koeda M, Takano A, Asai K, Suhara T et al (2005). Effects of dopaminergic and serotonergic manipulation on emotional processing: a pharmacological fMRI study. Neuroimage 27: 991–1001.
Takano A, Arakawa R, Hayashi M, Takahashi H, Ito H, Suhara T (2007). Relationship between neuroticism personality trait and serotonin transporter binding. Biol Psychiatry 62: 588–592.
Tangney JP (1990). Assessing individual-differences in proneness to shame and guilt – development of the self-conscious affect and attribution inventory. J Pers Soc Psychol 59: 102–111.
Tangney JP, Wagner P, Gramzow R (1992). Proneness to shame, proneness to guilt, and psychopathology. J Abnorm Psychol 101: 469–478.
Teasdale JD, Dent J (1987). Cognitive vulnerability to depression: an investigation of two hypotheses. Br J Clin Psychol 26 (Pt 2): 113–126.
Thase ME, Simons AD, McGeary J, Cahalane JF, Hughes C, Harden T et al (1992). Relapse after cognitive behavior therapy of depression: potential implications for longer courses of treatment. Am J Psychiatry 149: 1046–1052.
Thomas EJ (2009). Interactions between rumination and cognition in depression. University of Manchester PhD thesis.
Thompson RJ, Berenbaum H (2006). Shame reactions to everyday dilemmas are associated with depressive disorder. Cogn Ther Res 30: 415–425.
Timbremont B, Braet C (2004). Cognitive vulnerability in remitted depressed children and adolescents. Behav Res Ther 42: 423–437.
Tranter R, Bell D, Gutting P, Harmer C, Healy D, Anderson IM (2009). The effect of serotonergic and noradrenergic antidepressants on face emotion processing in depressed patients. J Affect Disord 118: 87–93.
van der Veen FM, Evers EA, Deutz NE, Schmitt JA (2007). Effects of acute tryptophan depletion on mood and facial emotion perception related brain activation and performance in healthy women with and without a family history of depression. Neuropsychopharmacology 32: 216–224.
van der Veen FM, Evers EA, van Deursen JA, Deutz NE, Backes WH, Schmitt JA (2006). Acute tryptophan depletion reduces activation in the right hippocampus during encoding in an episodic memory task. Neuroimage 31: 1188–1196.
Van Vreeswijk MF, De Wilde EJ (2004). Autobiographical memory specificity, psychopathology, depressed mood and the use of the Autobiographical Memory Test: a meta-analysis. Behav Res Ther 42: 731–743.
Viinikainen M, Jaaskelainen IP, Alexandrov Y, Balk MH, Autti T, Sams M (2010). Nonlinear relationship between emotional valence and brain activity: evidence of separate negative and positive valence dimensions. Hum Brain Mapp (In press).
Vuilleumier P, Armony JL, Driver J, Dolan RJ (2001). Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron 30: 829–841.
Vuilleumier P, Pourtois G (2007). Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia 45: 174–194.
Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ (2004). Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci 7: 1271–1278. Combined lesion and fMRI study of face processing with implications for understanding circuitry.
Wager TD, Phan KL, Liberzon I, Taylor SF (2003). Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage 19: 513–531.
Wagner V, Muller JL, Sommer M, Klein HE, Hajak G (2004). [Changes in the emotional processing in depressive patients: a study with functional magnetoresonance tomography under the employment of pictures with affective contents]. Psychiatr Prax 31 (Suppl 1): S70–S72.
Wang L, LaBar KS, Smoski M, Rosenthal MZ, Dolcos F, Lynch TR et al (2008). Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Res 163: 143–155.
Wang L, McCarthy G, Song AW, Labar KS (2005). Amygdala activation to sad pictures during high-field (4 tesla) functional magnetic resonance imaging. Emotion 5: 12–22.
Weiner B (1985). An attributional theory of achievement-motivation and emotion. Psychol Rev 92: 548–573.
Welt L (1888). Über Charakterveränderungen des Menschen. Dtsch Arch Klin Med 42: 339–390.
Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA et al (1998). The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry 44: 1219–1228.
Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL (2001). A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion 1: 70–83.
Williams JM, Broadbent K (1986). Autobiographical memory in suicide attempters. J Abnorm Psychol 95: 144–149.
Williams JM, Mathews A, MacLeod C (1996). The emotional Stroop task and psychopathology. Psychol Bull 120: 3–24.
Williams LM, Gatt JM, Schofield PR, Olivieri G, Peduto A, Gordon E (2009). Negativity bias' in risk for depression and anxiety: brain-body fear circuitry correlates 5-HTT-LPR and early life stress. Neuroimage 47: 804–814.
Winston JS, O'Doherty J, Dolan RJ (2003). Common and distinct neural responses during direct and incidental processing of multiple facial emotions. Neuroimage 20: 84–97.
Wittchen HU, Beesdo K, Bittner A, Goodwin RD (2003). Depressive episodes—evidence for a causal role of primary anxiety disorders? Eur Psychiatry 18: 384–393.
Young AW, Rowland D, Calder AJ, Etcoff NL, Seth A, Perrett DI (1997). Facial expression megamix: tests of dimensional and category accounts of emotion recognition. Cognition 63: 271–313.
Zahn R, de Oliveira-Souza R, Bramati I, Garrido G, Moll J (2009a). Subgenual cingulate activity reflects individual differences in empathic concern. Neurosci Lett 457: 107–110.
Zahn R, Moll J, Paiva M, Garrido G, Krueger F, Huey ED et al (2009b). The neural basis of human social values: evidence from functional MRI. Cereb Cortex 19: 276–283.
Acknowledgements
We thank our colleagues in the Neuroscience and Psychiatry Unit for their contribution to work discussed herein and Helen Mayberg for allowing us to adapt her model for Figure 4. Recent studies in our department were funded by the Medical Research Council and the EU (NewMood).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
RZ has received compensation from Pfizer for professional services unrelated to the contents of this review. IMA has received financial support from AstraZeneca and Servier for research unrelated to the contents of this review. JFWD has received financial support from Lilly, AstraZeneca and Servier for research unrelated to the contents of this review. RE has received financial support from Cambridge Cognition Limited for research unrelated to the contents of this review. The authors declare that, except for income received from their primary employer, no other financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Elliott, R., Zahn, R., Deakin, J. et al. Affective Cognition and its Disruption in Mood Disorders. Neuropsychopharmacol 36, 153–182 (2011). https://doi.org/10.1038/npp.2010.77
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2010.77
Keywords
This article is cited by
-
Functional brain responses to emotional faces after three to five weeks of intake of escitalopram in healthy individuals: a double-blind, placebo-controlled randomised study
Scientific Reports (2024)
-
The pharmacological bases for repurposing statins in depression: a review of mechanistic studies
Translational Psychiatry (2023)
-
Probing the antidepressant potential of psilocybin: integrating insight from human research and animal models towards an understanding of neural circuit mechanisms
Psychopharmacology (2023)
-
Shared and unique imaging-derived endo-phenotypes of two typical antidepressant-applicative depressive patients
European Radiology (2022)
-
The effects of social comparison and depressive mood on adolescent social decision-making
BMC Psychiatry (2021)