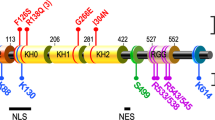
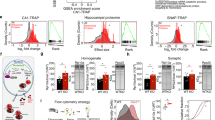
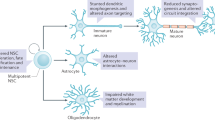
Abstract
De novo protein synthesis is necessary for long-lasting modifications in synaptic strength and dendritic spine dynamics that underlie cognition. Fragile X syndrome (FXS), characterized by intellectual disability and autistic behaviors, holds promise for revealing the molecular basis for these long-term changes in neuronal function. Loss of function of the fragile X mental retardation protein (FMRP) results in defects in synaptic plasticity and cognition in many models of the disease. FMRP is a polyribosome-associated RNA-binding protein that regulates the synthesis of a set of plasticity-reated proteins by stalling ribosomal translocation on target mRNAs. The recent identification of mRNA targets of FMRP and its upstream regulators, and the use of small molecules to stall ribosomes in the absence of FMRP, have the potential to be translated into new therapeutic avenues for the treatment of FXS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
13 May 2013
In the version of this article initially published online, a section heading read “Novel therapy for FXS directed at its molecular function.” The correct heading is “Novel therapy for FXS directed at FMRP function.” The error has been corrected for the print, PDF and HTML versions of this article.
References
Klann, E. & Sweatt, J.D. Altered protein synthesis is a trigger for long-term memory formation. Neurobiol. Learn. Mem. 89, 247–259 (2008).
Bhakar, A.L., Dolen, G. & Bear, M.F. The pathophysiology of fragile X (and what it teaches us about synapses). Annu. Rev. Neurosci. 35, 417–443 (2012).
Santoro, M.R., Bray, S.M. & Warren, S.T. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu. Rev. Pathol. 7, 219–245 (2012).
Gatto, C.L. & Broadie, K. The fragile X mental retardation protein in circadian rhythmicity and memory consolidation. Mol. Neurobiol. 39, 107–129 (2009).
Comery, T.A. et al. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc. Natl. Acad. Sci. USA 94, 5401–5404 (1997).
Nimchinsky, E.A., Oberlander, A.M. & Svoboda, K. Abnormal development of dendritic spines in FMR1 knock-out mice. J. Neurosci. 21, 5139–5146 (2001).
Cruz-Martin, A., Crespo, M. & Portera-Cailliau, C. Delayed stabilization of dendritic spines in fragile X mice. J. Neurosci. 30, 7793–7803 (2010).
Pan, F., Aldridge, G.M., Greenough, W.T. & Gan, W.B. Dendritic spine instability and insensitivity to modulation by sensory experience in a mouse model of fragile X syndrome. Proc. Natl. Acad. Sci. USA 107, 17768–17773 (2010).
Nosyreva, E.D. & Huber, K.M. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J. Neurophysiol. 95, 3291–3295 (2006).
Hou, L. et al. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron 51, 441–454 (2006).
Harlow, E.G. et al. Critical period plasticity is disrupted in the barrel cortex of FMR1 knockout mice. Neuron 65, 385–398 (2010).
Till, S.M. et al. Altered maturation of the primary somatosensory cortex in a mouse model of fragile X syndrome. Hum. Mol. Genet. 21, 2143–2156 (2012).
Christie, S.B., Akins, M.R., Schwob, J.E. & Fallon, J.R. The FXG: a presynaptic fragile X granule expressed in a subset of developing brain circuits. J. Neurosci. 29, 1514–1524 (2009).
Stefani, G., Fraser, C.E., Darnell, J.C. & Darnell, R.B. Fragile X mental retardation protein is associated with translating polyribosomes in neuronal cells. J. Neurosci. 24, 7272–7276 (2004).
Khandjian, E.W. et al. Biochemical evidence for the association of fragile X mental retardation protein with brain polyribosomal ribonucleoparticles. Proc. Natl. Acad. Sci. USA 101, 13357–13362 (2004).
Darnell, J.C. et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261 (2011).
Verkerk, A.J. et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65, 905–914 (1991).
Siomi, H., Siomi, M.C., Nussbaum, R.L. & Dreyfuss, G. The protein product of the fragile X gene, FMR1, has characteristics of an RNA-binding protein. Cell 74, 291–298 (1993).
De Boulle, K. et al. A point mutation in the FMR-1 gene associated with fragile X mental retardation. Nat. Genet. 3, 31–35 (1993).
Lewis, H.A. et al. Sequence-specific RNA binding by a Nova KH domain: implications for paraneoplastic disease and the fragile X syndrome. Cell 100, 323–332 (2000).
Feng, Y. et al. FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol. Cell 1, 109–118 (1997).
Zang, J.B. et al. A mouse model of the human Fragile X syndrome I304N mutation. PLoS Genet. 5, e1000758 (2009).
Bolduc, F.V., Bell, K., Cox, H., Broadie, K.S. & Tully, T. Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nat. Neurosci. 11, 1143–1145 (2008).
Ramos, A., Hollingworth, D. & Pastore, A. The role of a clinically important mutation in the fold and RNA-binding properties of KH motifs. RNA 9, 293–298 (2003).
Darnell, J.C. et al. Kissing complex RNAs mediate interaction between the Fragile-X mental retardation protein KH2 domain and brain polyribosomes. Genes Dev. 19, 903–918 (2005).
Ashley, C.T., Wilkinson, K.D., Reines, D. & Warren, S.T. FMR-1 protein: conserved RNP family domains and selective RNA binding. Science 262, 563–566 (1993).
Corbin, F. et al. The fragile X mental retardation protein is associated with poly(A)+ mRNA in actively translating polyribosomes. Hum. Mol. Genet. 6, 1465–1472 (1997).
Darnell, J.C. & Richter, J.D. Cytoplasmic RNA-binding proteins and the control of complex brain function. Cold Spring Harb. Perspect. Biol. 4, a012344 (2012).
Darnell, R.B. HITS-CLIP: panoramic views of protein-RNA regulation in living cells. Wiley Interdiscip. Rev. RNA 1, 266–286 (2010).
Shanks, N.F. et al. Differences in AMPA and kainate receptor interactomes facilitate identification of AMPA receptor auxiliary subunit GSG1L. Cell Rep. 1, 590–598 (2012).
Akins, M.R., Berk-Rauch, H.E. & Fallon, J.R. Presynaptic translation: stepping out of the postsynaptic shadow. Front. Neural Circuits 3, 17 (2009).
Hanson, J.E. & Madison, D.V. Presynaptic FMR1 genotype influences the degree of synaptic connectivity in a mosaic mouse model of fragile X syndrome. J. Neurosci. 27, 4014–4018 (2007).
Deng, P.Y., Sojka, D. & Klyachko, V.A. Abnormal presynaptic short-term plasticity and information processing in a mouse model of fragile X syndrome. J. Neurosci. 31, 10971–10982 (2011).
Zhang, J., Hou, L., Klann, E. & Nelson, D.L. Altered hippocampal synaptic plasticity in the FMR1 gene family knockout mouse models. J. Neurophysiol. 101, 2572–2580 (2009).
Gibson, J.R., Bartley, A.F., Hays, S.A. & Huber, K.M. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J. Neurophysiol. 100, 2615–2626 (2008).
Suvrathan, A., Hoeffer, C.A., Wong, H., Klann, E. & Chattarji, S. Characterization and reversal of synaptic defects in the amygdala in a mouse model of fragile X syndrome. Proc. Natl. Acad. Sci. USA 107, 11591–11596 (2010).
Sebeo, J. et al. Requirement for protein synthesis at developing synapses. J. Neurosci. 29, 9778–9793 (2009).
Antar, L.N., Li, C., Zhang, H., Carroll, R.C. & Bassell, G.J. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol. Cell Neurosci. 32, 37–48 (2006).
Laggerbauer, B., Ostareck, D., Keidel, E.M., Ostareck-Lederer, A. & Fischer, U. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum. Mol. Genet. 10, 329–338 (2001).
Li, Z. et al. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res. 29, 2276–2283 (2001).
Gross, C. et al. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J. Neurosci. 30, 10624–10638 (2010).
Gross, C. & Bassell, G.J. Excess protein synthesis in FXS patient lymphoblastoid cells can be rescued with a p110beta-selective inhibitor. Mol. Med. 18, 336–345 (2012).
Dölen, G. et al. Correction of fragile X syndrome in mice. Neuron 56, 955–962 (2007).
Bhattacharya, A. et al. Genetic removal of p70 S6 kinase 1 corrects molecular, synaptic and behavioral phenotypes in Fragile X syndrome mice. Neuron 76, 325–337 (2012).
Qin, M., Kang, J., Burlin, T.V., Jiang, C. & Smith, C.B. Postadolescent changes in regional cerebral protein synthesis: an in vivo study in the FMR1 null mouse. J. Neurosci. 25, 5087–5095 (2005).
Osterweil, E.K., Krueger, D.D., Reinhold, K. & Bear, M.F. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J. Neurosci. 30, 15616–15627 (2010).
Zhang, Y.Q. et al. Drosophila fragile X–related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell 107, 591–603 (2001).
Zalfa, F. et al. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell 112, 317–327 (2003).
Goebel-Goody, S.M. et al. Genetic manipulation of STEP reverses behavioral abnormalities in a fragile X syndrome mouse model. Genes Brain Behav. 11, 586–600 (2012).
Strumbos, J.G., Brown, M.R., Kronengold, J., Polley, D.B. & Kaczmarek, L.K. Fragile X mental retardation protein is required for rapid experience-dependent regulation of the potassium channel Kv3.1b. J. Neurosci. 30, 10263–10271 (2010).
Lee, H.Y. et al. Bidirectional regulation of dendritic voltage-gated potassium channels by the fragile X mental retardation protein. Neuron 72, 630–642 (2011).
Sharma, A. et al. Dysregulation of mTOR signaling in fragile X syndrome. J. Neurosci. 30, 694–702 (2010).
Schütt, J., Falley, K., Richter, D., Kreienkamp, H.J. & Kindler, S. Fragile X mental retardation protein regulates the levels of scaffold proteins and glutamate receptors in postsynaptic densities. J. Biol. Chem. 284, 25479–25487 (2009).
Hoeffer, C.A. et al. Altered mTOR signaling and enhanced CYFIP2 expression levels in subjects with fragile X syndrome. Genes Brain Behav. 11, 332–341 (2012).
Krueger, D.D., Osterweil, E.K., Chen, S.P., Tye, L.D. & Bear, M.F. Cognitive dysfunction and prefrontal synaptic abnormalities in a mouse model of fragile X syndrome. Proc. Natl. Acad. Sci. USA 108, 2587–2592 (2011).
Bear, M.F., Huber, K.M. & Warren, S.T. The mGluR theory of fragile X mental retardation. Trends Neurosci. 27, 370–377 (2004).
Michalon, A. et al. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron 74, 49–56 (2012).
Berry-Kravis, E. et al. A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. J. Med. Genet. 46, 266–271 (2009).
Klann, E. & Dever, T.E. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat. Rev. Neurosci. 5, 931–942 (2004).
Hoeffer, C.A. & Klann, E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 33, 67–75 (2010).
Kelleher, R.J.r. & Bear, M.F. The autistic neuron: troubled translation? Cell 135, 401–406 (2008).
Kelley, D.J. et al. The cyclic AMP cascade is altered in the fragile X nervous system. PLoS ONE 2, e931 (2007).
Berry-Kravis, E. & Sklena, P. Demonstration of abnormal cyclic AMP production in platelets from patients with fragile X syndrome. Am. J. Med. Genet. 45, 81–87 (1993).
Li, J., Pelletier, M.R., Perez Velazquez, J.L. & Carlen, P.L. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol. Cell Neurosci. 19, 138–151 (2002).
Newey, S.E., Velamoor, V., Govek, E.E. & Van Aelst, L. Rho GTPases, dendritic structure and mental retardation. J. Neurobiol. 64, 58–74 (2005).
Rumbaugh, G., Adams, J.P., Kim, J.H. & Huganir, R.L. SynGAP regulates synaptic strength and mitogen-activated protein kinases in cultured neurons. Proc. Natl. Acad. Sci. USA 103, 4344–4351 (2006).
Pinto, D. et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature 466, 368–372 (2010).
Huber, K.M., Kayser, M.S. & Bear, M.F. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288, 1254–1257 (2000).
Huber, K.M., Gallagher, S.M., Warren, S.T. & Bear, M.F. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. USA 99, 7746–7750 (2002).
McBride, S.M. et al. Pharmacological rescue of synaptic plasticity, courtship behavior and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 45, 753–764 (2005).
El Idrissi, A. et al. Decreased GABA(A) receptor expression in the seizure-prone fragile X mouse. Neurosci. Lett. 377, 141–146 (2005).
D'Hulst, C. et al. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 1121, 238–245 (2006).
Adusei, D.C., Pacey, L.K., Chen, D. & Hampson, D.R. Early developmental alterations in GABAergic protein expression in fragile X knockout mice. Neuropharmacology 59, 167–171 (2010).
Chang, S. et al. Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat. Chem. Biol. 4, 256–263 (2008).
Heulens, I., D'Hulst, C., Van Dam, D., De Deyn, P.P. & Kooy, R.F. Pharmacological treatment of fragile X syndrome with GABAergic drugs in a knockout mouse model. Behav. Brain Res. 229, 244–249 (2012).
Henderson, C. et al. Reversal of disease-related pathologies in the fragile X mouse model by selective activation of GABA(B) receptors with arbaclofen. Sci. Transl. Med. 4, 152ra128 (2012).
Berry-Kravis, E.M. et al. Effects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: a randomized, controlled, phase 2 trial. Sci. Transl. Med. 4, 152ra127 (2012).
Richter, J.D. & Klann, E. Making synaptic plasticity and memory last: mechanisms of translational regulation. Genes Dev. 23, 1–11 (2009).
Klann, E., Antion, M.D., Banko, J.L. & Hou, L. Synaptic plasticity and translation initiation. Learn. Mem. 11, 365–372 (2004).
Costa-Mattioli, M., Sossin, W.S., Klann, E. & Sonenberg, N. Translational control of long-lasting synaptic plasticity and memory. Neuron 61, 10–26 (2009).
Ronesi, J.A. et al. Disrupted Homer scaffolds mediate abnormal mGluR5 function in a mouse model of fragile X syndrome. Nat. Neurosci. 15, 431–440 (2012).
Romanelli, A., Martin, K.A., Toker, A. & Blenis, J. p70 S6 kinase is regulated by protein kinase Czeta and participates in a phosphoinositide 3-kinase–regulated signaling complex. Mol. Cell Biol. 19, 2921–2928 (1999).
Osterweil, E.K. et al. Lovastatin corrects excess protein synthesis and prevents epileptogenesis in a mouse model of fragile X syndrome. Neuron 77, 243–250 (2013).
Kwiatkowski, D.J. & Manning, B.D. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum. Mol. Genet. 14, 251–258 (2005).
Auerbach, B.D., Osterweil, E.K. & Bear, M.F. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature 480, 63–68 (2011).
Ozcan, U. et al. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol. Cell 29, 541–551 (2008).
Mines, M.A., Yuskaitis, C.J., King, M.K., Beurel, E. & Jope, R.S. GSK3 influences social preference and anxiety-related behaviors during social interaction in a mouse model of fragile X syndrome and autism. PLoS ONE 5, e9706 (2010).
Guo, W. et al. Inhibition of GSK3beta improves hippocampus-dependent learning and rescues neurogenesis in a mouse model of fragile X syndrome. Hum. Mol. Genet. 21, 681–691 (2012).
Ronesi, J.A. & Huber, K.M. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J. Neurosci. 28, 543–547 (2008).
Neves-Pereira, M. et al. Deregulation of EIF4E: a novel mechanism for autism. J. Med. Genet. 46, 759–765 (2009).
Napoli, I. et al. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell 134, 1042–1054 (2008).
Santini, E. et al. Exaggerated translation causes synaptic and behavioural aberrations associated with autism. Nature 493, 411–415 (2013).
Bilousova, T.V. et al. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J. Med. Genet. 46, 94–102 (2009).
Siller, S.S. & Broadie, K. Neural circuit architecture defects in a Drosophila model of Fragile X syndrome are alleviated by minocycline treatment and genetic removal of matrix metalloproteinase. Dis. Model Mech. 4, 673–685 (2011).
Kim, H.S. & Suh, Y.H. Minocycline and neurodegenerative diseases. Behav. Brain Res. 196, 168–179 (2009).
Budkevich, T.V., El'skaya, A.V. & Nierhaus, K.H. Features of 80S mammalian ribosome and its subunits. Nucleic Acids Res. 36, 4736–4744 (2008).
Paribello, C. et al. Open-label add-on treatment trial of minocycline in fragile X syndrome. BMC Neurol. 10, 91 (2010).
Acknowledgements
This work was supported by US National Institutes of Health grants HD040647 (J.C.D.) and NS047384 (E.K.), and Congressionally Directed Medical Research Program Award W81-XWH-11-1-0389 from the US Department of Defense (E.K.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Source data
Rights and permissions
About this article
Cite this article
Darnell, J., Klann, E. The translation of translational control by FMRP: therapeutic targets for FXS. Nat Neurosci 16, 1530–1536 (2013). https://doi.org/10.1038/nn.3379
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3379
This article is cited by
-
FMRP binds Per1 mRNA and downregulates its protein expression in mice
Molecular Brain (2023)
-
Role of the endocannabinoid system in fragile X syndrome: potential mechanisms for benefit from cannabidiol treatment
Journal of Neurodevelopmental Disorders (2023)
-
Phenotypic variability to medication management: an update on fragile X syndrome
Human Genomics (2023)
-
Caveolin-1-Mediated Cholesterol Accumulation Contributes to Exaggerated mGluR-Dependent Long-Term Depression and Impaired Cognition in Fmr1 Knockout Mice
Molecular Neurobiology (2023)
-
Psilocybin mitigates the cognitive deficits observed in a rat model of Fragile X syndrome
Psychopharmacology (2023)