Abstract
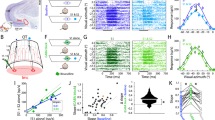
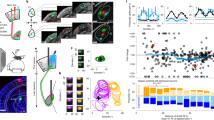
The mechanisms by which the brain suppresses distracting stimuli to control the locus of attention are unknown. We found that focal, reversible inactivation of a single inhibitory circuit in the barn owl midbrain tegmentum, the nucleus isthmi pars magnocellularis (Imc), abolished both stimulus-driven (exogenous) and internally driven (endogenous) competitive interactions in the optic tectum (superior colliculus in mammals), which are vital to the selection of a target among distractors in behaving animals. Imc neurons transformed spatially precise multisensory and endogenous input into powerful inhibitory output that suppressed competing representations across the entire tectal space map. We identified a small, but highly potent, circuit that is employed by both exogenous and endogenous signals to exert competitive suppression in the midbrain selection network. Our findings reveal, to the best of our knowledge, for the first time, a neural mechanism for the construction of a priority map that is critical for the selection of the most important stimulus for gaze and attention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Fecteau, J.H. & Munoz, D.P. Salience, relevance, and firing: a priority map for target selection. Trends Cogn. Sci. 10, 382–390 (2006).
Awh, E., Belopolsky, A.V. & Theeuwes, J. Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends Cogn. Sci. 16, 437–443 (2012).
Reynolds, J.H. & Chelazzi, L. Attentional modulation of visual processing. Annu. Rev. Neurosci. 27, 611–647 (2004).
Bisley, J.W. The neural basis of visual attention. J. Physiol. (Lond.) 589, 49–57 (2011).
Gazzaley, A., Cooney, J.W., Rissman, J. & D′Esposito, M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat. Neurosci. 8, 1298–1300 (2005).
McMains, S. & Kastner, S. Interactions of top-down and bottom-up mechanisms in human visual cortex. J. Neurosci. 31, 587–597 (2011).
Knudsen, E.I. Control form below: the contribution of a midbrain network to spatial attention. Eur. J. Neurosci. 33, 1961–1972 (2011).
Zanto, T.P. & Gazzaley, A. Neural suppression of irrelevant information underlies optimal working memory performance. J. Neurosci. 29, 3059–3066 (2009).
Lovejoy, L.P. & Krauzlis, R.J. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat. Neurosci. 13, 261–266 (2010).
McPeek, R.M. & Keller, E.L. Deficits in saccade target selection after inactivation of superior colliculus. Nat. Neurosci. 7, 757–763 (2004).
Mysore, S.P., Asadollahi, A. & Knudsen, E.I. Global inhibition and stimulus competition in the owl optic tectum. J. Neurosci. 30, 1727–1738 (2010).
Winkowski, D.E. & Knudsen, E.I. Top-down gain control of the auditory space map by gaze control circuitry in the barn owl. Nature 439, 336–339 (2006).
Mysore, S.P., Asadollahi, A. & Knudsen, E.I. Signaling of the strongest stimulus in the owl optic tectum. J. Neurosci. 31, 5186–5196 (2011).
Basso, M.A. & Wurtz, R.H. Modulation of neuronal activity by target uncertainty. Nature 389, 66–69 (1997).
Mysore, S.P. & Knudsen, E.I. Flexible categorization of relative stimulus strength by the optic tectum. J. Neurosci. 31, 7745–7752 (2011).
Mysore, S.P. & Knudsen, E.I. The role of a midbrain network in competitive stimulus selection. Curr. Opin. Neurobiol. 21, 653–660 (2011).
Carrasco, M. Covert attention increases contrast sensitivity: psychophysical, neurophysiological and neuroimaging studies. Prog. Brain Res. 154, 33–70 (2006).
Wang, Y., Major, D.E. & Karten, H.J. Morphology and connections of nucleus isthmi pars magnocellularis in chicks (Gallus gallus). J. Comp. Neurol. 469, 275–297 (2004).
Marín, G. et al. A cholinergic gating mechanism controlled by competitive interactions in the optic tectum of the pigeon. J. Neurosci. 27, 8112–8121 (2007).
Knudsen, E.I., Cohen, Y.E. & Masino, T. Characterization of a forebrain gaze field in the archistriatum of the barn owl: microstimulation and anatomical connections. J. Neurosci. 15, 5139–5151 (1995).
Stanton, G.B., Goldberg, M.E. & Bruce, C.J. Frontal eye field efferents in the macaque monkey. II. Topography of terminal fields in midbrain and pons. J. Comp. Neurol. 271, 493–506 (1988).
Knudsen, E.I. & Knudsen, P.F. Disruption of auditory spatial working memory by inactivation of the forebrain archistriatum in barn owls. Nature 383, 428–431 (1996).
Dias, E.C. & Segraves, M.A. Muscimol-induced inactivation of monkey frontal eye field: effects on visually and memory-guided saccades. J. Neurophysiol. 81, 2191–2214 (1999).
Bruce, C.J., Goldberg, M.E., Bushnell, M.C. & Stanton, G.B. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J. Neurophysiol. 54, 714–734 (1985).
Moore, T. & Armstrong, K.M. Selective gating of visual signals by microstimulation of frontal cortex. Nature 421, 370–373 (2003).
Moore, T. & Fallah, M. Control of eye movements and spatial attention. Proc. Natl. Acad. Sci. USA 98, 1273–1276 (2001).
Sundberg, K.A., Mitchell, J.F. & Reynolds, J.H. Spatial attention modulates center-surround interactions in macaque visual area v4. Neuron 61, 952–963 (2009).
Kastner, S., Pinsk, M.A., De Weerd, P., Desimone, R. & Ungerleider, L.G. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22, 751–761 (1999).
Falkner, A.L., Krishna, B.S. & Goldberg, M.E. Surround suppression sharpens the priority map in the lateral intraparietal area. J. Neurosci. 30, 12787–12797 (2010).
Bair, W., Cavanaugh, J.R. & Movshon, J.A. Time course and time-distance relationships for surround suppression in macaque V1 neurons. J. Neurosci. 23, 7690–7701 (2003).
Schall, J.D., Hanes, D.P., Thompson, K.G. & King, D.J. Saccade target selection in frontal eye field of macaque. I. Visual and premovement activation. J. Neurosci. 15, 6905–6918 (1995).
Desimone, R., Moran, J., Schein, S.J. & Mishkin, M. A role for the corpus callosum in visual area V4 of the macaque. Vis. Neurosci. 10, 159–171 (1993).
Desimone, R. & Duncan, J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 18, 193–222 (1995).
Knudsen, E.I. Fundamental components of attention. Annu. Rev. Neurosci. 30, 57–78 (2007).
Nummela, S.U. & Krauzlis, R.J. Inactivation of primate superior colliculus biases target choice for smooth pursuit, saccades and button press responses. J. Neurophysiol. 104, 1538–1548 (2010).
Zénon, A. & Krauzlis, R.J. Attention deficits without cortical neuronal deficits. Nature 489, 434–437 (2012).
Marín, G., Mpodozis, J., Sentis, E., Ossandon, T. & Letelier, J.C. Oscillatory bursts in the optic tectum of birds represent re-entrant signals from the nucleus isthmi pars parvocellularis. J. Neurosci. 25, 7081–7089 (2005).
Lee, D.K., Itti, L., Koch, C. & Braun, J. Attention activates winner-take-all competition among visual filters. Nat. Neurosci. 2, 375–381 (1999).
Reynolds, J.H. & Heeger, D.J. The normalization model of attention. Neuron 61, 168–185 (2009).
Lee, J. & Maunsell, J.H. A normalization model of attentional modulation of single unit responses. PLoS ONE 4, e4651 (2009).
Cavanaugh, J.R., Bair, W. & Movshon, J.A. Nature and interaction of signals from the receptive field center and surround in macaque V1 neurons. J. Neurophysiol. 88, 2530–2546 (2002).
Kastner, S. & Ungerleider, L.G. Mechanisms of visual attention in the human cortex. Annu. Rev. Neurosci. 23, 315–341 (2000).
Carrasco, M. Visual attention: the past 25 years. Vision Res. 51, 1484–1525 (2011).
Gazzaley, A., Cooney, J.W., McEvoy, K., Knight, R.T. & D′Esposito, M. Top-down enhancement and suppression of the magnitude and speed of neural activity. J. Cogn. Neurosci. 17, 507–517 (2005).
Winkowski, D.E. & Knudsen, E.I. Distinct mechanisms for top-down control of neural gain and sensitivity in the owl optic tectum. Neuron 60, 698–708 (2008).
Mysore, S.P. & Knudsen, E.I. Reciprocal inhibition of inhibition: a circuit motif for flexible categorization in stimulus selection. Neuron 73, 193–205 (2012).
Witten, I.B., Knudsen, P.F. & Knudsen, E.I. A dominance hierarchy of auditory spatial cues in barn owls. PLoS ONE 5, e10396 (2010).
Winkowski, D.E. & Knudsen, E.I. Top-down control of multimodal sensitivity in the barn owl optic tectum. J. Neurosci. 27, 13279–13291 (2007).
Acknowledgements
We thank P. Knudsen for the immunohistochemistry. We are grateful to A. Asadollahi, A. Bryant, A. Goddard, J. Schwarz and N. Steinmetz for critically reading the manuscript. This work was supported by funding from the US National Institutes of Health (9R01 EY019179, E.I.K.).
Author information
Authors and Affiliations
Contributions
S.P.M. and E.I.K. designed the study and wrote the paper. S.P.M. performed the experiments and the analyses.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 2185 kb)
Rights and permissions
About this article
Cite this article
Mysore, S., Knudsen, E. A shared inhibitory circuit for both exogenous and endogenous control of stimulus selection. Nat Neurosci 16, 473–478 (2013). https://doi.org/10.1038/nn.3352
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3352
This article is cited by
-
Interhemispheric competition during sleep
Nature (2023)
-
Superior colliculus bidirectionally modulates choice activity in frontal cortex
Nature Communications (2023)
-
Distinct neural mechanisms construct classical versus extraclassical inhibitory surrounds in an inhibitory nucleus in the midbrain attention network
Nature Communications (2023)
-
Neuronal modulation in the mouse superior colliculus during covert visual selective attention
Scientific Reports (2022)
-
Donut-like organization of inhibition underlies categorical neural responses in the midbrain
Nature Communications (2022)