Abstract
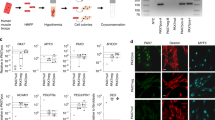
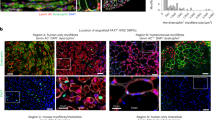
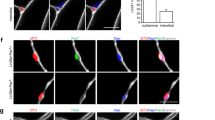
Little progress has been made toward the use of embryonic stem (ES) cells to study and isolate skeletal muscle progenitors. This is due to the paucity of paraxial mesoderm formation during embryoid body (EB) in vitro differentiation and to the lack of reliable identification and isolation criteria for skeletal muscle precursors. Here we show that expression of the transcription factor Pax3 during embryoid body differentiation enhances both paraxial mesoderm formation and the myogenic potential of the cells within this population. Transplantation of Pax3-induced cells results in teratomas, however, indicating the presence of residual undifferentiated cells. By sorting for the PDGF-α receptor, a marker of paraxial mesoderm, and for the absence of Flk-1, a marker of lateral plate mesoderm, we derive a cell population from differentiating ES cell cultures that has substantial muscle regeneration potential. Intramuscular and systemic transplantation of these cells into dystrophic mice results in extensive engraftment of adult myofibers with enhanced contractile function without the formation of teratomas. These data demonstrate the therapeutic potential of ES cells in muscular dystrophy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Robertson, E.J. Pluripotential stem cell lines as a route into the mouse germ line. Trends Genet. 2, 9–13 (1986).
Rohwedel, J. et al. Muscle cell differentiation of embryonic stem cells reflects myogenesis in vivo: developmentally regulated expression of myogenic determination genes and functional expression of ionic currents. Dev. Biol. 164, 87–101 (1994).
Weitzer, G., Milner, D.J., Kim, J.U., Bradley, A. & Capetanaki, Y. Cytoskeletal control of myogenesis: a desmin null mutation blocks the myogenic pathway during embryonic stem cell differentiation. Dev. Biol. 172, 422–439 (1995).
Ridgeway, A.G. & Skerjanc, I.S. Pax3 is essential for skeletal myogenesis and the expression of Six1 and Eya2. J. Biol. Chem. 276, 19033–19039 (2001).
Armour, C., Garson, K. & McBurney, M.W. Cell-cell interaction modulates myoD-induced skeletal myogenesis of pluripotent P19 cells in vitro. Exp. Cell Res. 251, 79–91 (1999).
Bhagavati, S. & Xu, W. Generation of skeletal muscle from transplanted embryonic stem cells in dystrophic mice. Biochem. Biophys. Res. Commun. 333, 644–649 (2005).
Barberi, T. et al. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat. Med. 13, 642–648 (2007).
Christ, B. & Ordahl, C.P. Early stages of chick somite development. Anat. Embryol. (Berl.) 191, 381–396 (1995).
Bailey, P., Holowacz, T. & Lassar, A.B. The origin of skeletal muscle stem cells in the embryo and the adult. Curr. Opin. Cell Biol. 13, 679–689 (2001).
Goulding, M., Lumsden, A. & Paquette, A.J. Regulation of Pax-3 expression in the dermomyotome and its role in muscle development. Development 120, 957–971 (1994).
Denetclaw, W.F.J., Christ, B. & Ordahl, C.P. Location and growth of epaxial myotome precursor cells. Development 124, 1601–1610 (1997).
Ordahl, C.P. & Le Douarin, N.M. Two myogenic lineages within the developing somite. Development 114, 339–353 (1992).
Maroto, M. et al. Ectopic Pax-3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue. Cell 89, 139–148 (1997).
Bajard, L. et al. A novel genetic hierarchy functions during hypaxial myogenesis: Pax3 directly activates Myf5 in muscle progenitor cells in the limb. Genes Dev. 20, 2450–2464 (2006).
Relaix, F., Rocancourt, D., Mansouri, A. & Buckingham, M.A. Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 435, 948–953 (2005).
Kassar-Duchossoy, L. et al. Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev. 19, 1426–1431 (2005).
Kyba, M., Perlingeiro, R.C. & Daley, G.Q. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell 109, 29–37 (2002).
Fehling, H.J. et al. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development 130, 4217–4227 (2003).
Lois, C., Hong, E.J., Pease, S., Brown, E.J. & Baltimore, D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295, 868–872 (2002).
Ema, M., Takahashi, S. & Rossant, J. Deletion of the selection cassette, but not cis-acting elements, in targeted Flk-1-lacZ allele reveals Flk-1 expression in multipotent mesodermal progenitors. Blood 107, 111–117 (2006).
Takakura, N. et al. PDGFRα expression during mouse embryogenesis: immunolocalization analyzed by whole-mount immunohistostaining using the monoclonal anti-mouse PDGFRα antibody APA5. J. Histochem. Cytochem. 45, 883–893 (1997).
Sakurai, H. et al. In vitro modeling of paraxial and lateral mesoderm differentiation reveals early reversibility. Stem Cells 24, 575–586 (2006).
Tortorella, L.L., Milasincic, D.J. & Pilch, P.F. Critical proliferation-independent window for basic fibroblast growth factor repression of myogenesis via the p42/p44 MAPK signaling pathway. J. Biol. Chem. 276, 13709–13717 (2001).
Sampaolesi, M. et al. Cell therapy of α-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science 301, 487–492 (2003).
Bachrach, E. et al. Muscle engraftment of myogenic progenitor cells following intraarterial transplantation. Muscle Nerve 34, 44–52 (2006).
Lin, J. et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 418, 797–801 (2002).
Partridge, T.A., Morgan, J.E., Coulton, G.R., Hoffman, E.P. & Kunkel, L.M. Conversion of mdx myofibers from dystrophin-negative to positive by injection of normal myoblasts. Nature 337, 176–179 (1989).
Gussoni, E., Blau, H.M. & Kunkel, L.M. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nat. Med. 3, 970–977 (1997).
Qu, Z. et al. Development of approaches to improve cell survival in myoblast transfer therapy. J. Cell Biol. 142, 1257–1267 (1998).
Tremblay, J.P. et al. Results of a triple blind clinical study of myoblast transplantations without immunosuppressive treatment in young boys with Duchenne muscular dystrophy. Cell Transplant. 2, 99–112 (1993).
Mendell, J.R. et al. Myoblast transfer in the treatment of Duchenne's muscular dystrophy. N. Engl. J. Med. 333, 832–838 (1995).
Partridge, T., Lu, Q.L., Morris, G. & Hoffman, E. Is myoblast transplantation effective? Nat. Med. 4, 1208–1209 (1998).
Asakura, A., Seale, P., Girgis-Gabardo, A. & Rudnicki, M.A. Myogenic specification of side population cells in skeletal muscle. J. Cell Biol. 159, 123–134 (2002).
Rando, T.A. & Blau, H.M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 125, 1275–1287 (1994).
Lee, J.Y. et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J. Cell Biol. 150, 1085–1100 (2000).
Sampaolesi, M. et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature 444, 574–579 (2006).
Montarras, D. et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science 309, 2064–2067 (2005).
Sherwood, R.I. et al. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell 119, 543–554 (2004).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science published online, doi:10.1126/science.1151526 (20 November 2007).
Fujikawa, T. et al. Teratoma formation leads to failure of treatment for type I diabetes using embryonic stem cell–derived insulin-producing cells. Am. J. Pathol. 166, 1781–1791 (2005).
Eventov-Friedman, S. et al. Embryonic pig liver, pancreas, and lung as a source for transplantation: optimal organogenesis without teratoma depends on distinct time windows. Proc. Natl. Acad. Sci. USA 102, 2928–2933 (2005).
Cornelison, D.D. & Wold, B.J. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev. Biol. 191, 270–283 (1997).
Cornelison, D.D., Filla, M.S., Stanley, H.M., Rapraeger, A.C. & Olwin, B.B. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev. Biol. 239, 79–94 (2001).
Sherwood, R.I., Christensen, J.L., Weissman, I.L. & Wagers, A.J. Determinants of skeletal muscle contributions from circulating cells, bone marrow cells, and hematopoietic stem cells. Stem Cells 22, 1292–1304 (2004).
Beauchamp, J.R. et al. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J. Cell Biol. 151, 1221–1234 (2000).
Kuang, S., Kuroda, K., Le Grand, F. & Rudnicki, M.A. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129, 999–1010 (2007).
Yoshimura, M. et al. AAV vector-mediated microdystrophin expression in a relatively small percentage of mdx myofibers improved the mdx phenotype. Mol. Ther. 10, 821–828 (2004).
Gross, J.G. & Morgan, J.E. Muscle precursor cells injected into irradiated mdx mouse muscle persist after serial injury. Muscle Nerve 22, 174–185 (1999).
Acknowledgements
We thank J. Stull for discussion and M. Rudnicki for technical advice. The monoclonal antibody to MHC was obtained from the Developmental Studies Hybridoma Bank, which was developed under the auspices of the National Institute of Child Health and Human Development and is maintained by the University of Iowa. This work was supported by the Dr. Bob and Jean Smith Foundation.
Author information
Authors and Affiliations
Contributions
R.D. designed and conducted the in vitro and in vivo experiments with iPax3 ES cells. R.D. also performed the final analysis of the data and contributed to writing the paper. K.G. generated the iPax3 ES cell line. R.M.B. performed the i.a. transplantations. S.K. performed quantifications for dystrophin and assisted R.D. with some of the staining. M.O. performed the i.v. transplantations. K.E.K. supervised and assisted on interpretation of the muscle functional analyses. M.K. provided materials, supervised K.G. on generating the cell line and analyzed the data. R.C.R.P. supervised the overall project, designed experiments, analyzed the data and wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Table 1 and Supplementary Methods (PDF 928 kb)
Rights and permissions
About this article
Cite this article
Darabi, R., Gehlbach, K., Bachoo, R. et al. Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nat Med 14, 134–143 (2008). https://doi.org/10.1038/nm1705
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1705
This article is cited by
-
Extracellular matrix: the critical contributor to skeletal muscle regeneration—a comprehensive review
Inflammation and Regeneration (2023)
-
Transplantation of PSC-derived myogenic progenitors counteracts disease phenotypes in FSHD mice
npj Regenerative Medicine (2022)
-
Preservation of satellite cell number and regenerative potential with age reveals locomotory muscle bias
Skeletal Muscle (2021)
-
Master regulators of skeletal muscle lineage development and pluripotent stem cells differentiation
Cell Regeneration (2021)
-
Approaches to characterize the transcriptional trajectory of human myogenesis
Cellular and Molecular Life Sciences (2021)