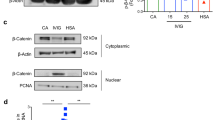
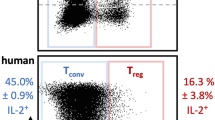
Abstract
Despite a more than 20-year experience of therapeutic benefit, the relevant molecular and cellular targets of intravenous immunoglobulin (IVIg) in autoimmune disease remain unclear. Contrary to the prevailing theories of IVIg action in autoimmunity, we show that IVIg drives signaling through activating Fcγ receptors (FcγR) in the amelioration of mouse immune thrombocytopenic purpura (ITP). The actual administration of IVIg was unnecessary because as few as 105 IVIg-treated cells could, upon adoptive transfer, ameliorate ITP. IVIg did not interact with the inhibitory FcγRIIB on the initiator cell, although FcγRIIB does have a role in the late phase of IVIg action. Notably, only IVIg-treated CD11c+ dendritic cells could mediate these effects. We hypothesize that IVIg forms soluble immune complexes in vivo that prime dendritic-cell regulatory activity. In conclusion, the clinical effects of IVIg in ameliorating ITP seem to involve the acute interaction of IVIg with activating FcγR on dendritic cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Knezevic-Maramica, I. & Kruskall, M.S. Intravenous immune globulins: an update for clinicians. Transfusion 43, 1460–1480 (2003).
Jolles, S., Sewell, W.A. & Misbah, S.A. Clinical uses of intravenous immunoglobulin. Clin. Exp. Immunol. 142, 1–11 (2005).
McMillan, R., Longmire, R.L., Yelenosky, R., Donnell, R.L. & Armstrong, S. Quantitation of platelet-binding IgG produced in vitro by spleens from patients with idiopathic thrombocytopenic purpura. N. Engl. J. Med. 291, 812–817 (1974).
Saleh, M.N., Moore, D.L., Lee, J.Y. & LoBuglio, A.F. Monocyte-platelet interaction in immune and nonimmune thrombocytopenia. Blood 74, 1328–1331 (1989).
Semple, J.W. Immune pathophysiology of autoimmune thrombocytopenic purpura. Blood Rev. 16, 9–12 (2002).
Bussel, J.B. Fc receptor blockade and immune thrombocytopenic purpura. Semin. Hematol. 37, 261–266 (2000).
Fehr, J., Hofmann, V. & Kappeler, U. Transient reversal of thrombocytopenia in idiopathic thrombocytopenic purpura by high-dose intravenous gamma globulin. N. Engl. J. Med. 306, 1254–1258 (1982).
Salama, A., Mueller-Eckhardt, C. & Kiefel, V. Effect of intravenous immunoglobulin in immune thrombocytopenia. Lancet 2, 193–195 (1983).
Salama, A., Kiefel, V., Amberg, R. & Mueller-Eckhardt, C. Treatment of autoimmune thrombocytopenic purpura with rhesus antibodies (anti-Rh0(D)). Blut 49, 29–35 (1984).
Lazarus, A.H. & Crow, A.R. Mechanism of action of IVIG and anti-D in ITP. Transfus. Apher. Sci. 28, 249–255 (2003).
Samuelsson, A., Towers, T.L. & Ravetch, J.V. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 291, 484–486 (2001).
Crow, A.R. et al. IVIg-mediated amelioration of murine ITP via Fc{gamma}RIIB is independent of SHIP1, SHP-1, and Btk activity. Blood 102, 558–560 (2003).
Siragam, V. et al. Can antibodies with specificity for soluble antigens mimic the therapeutic effects of intravenous IgG in the treatment of autoimmune disease? J. Clin. Invest. 115, 155–160 (2005).
Korganow, A.S. et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity 10, 451–461 (1999).
Kouskoff, V. et al. Organ-specific disease provoked by systemic autoimmunity. Cell 87, 811–822 (1996).
Bruhns, P., Samuelsson, A., Pollard, J.W. & Ravetch, J.V. Colony-stimulating factor-1-dependent macrophages are responsible for IVIG protection in antibody-induced autoimmune disease. Immunity 18, 573–581 (2003).
Cao, X. et al. The inositol 3-phosphatase PTEN negatively regulates Fc gamma receptor signaling, but supports Toll-like receptor 4 signaling in murine peritoneal macrophages. J. Immunol. 172, 4851–4857 (2004).
Latour, S., Bonnerot, C., Fridman, W.H. & Daeron, M. Induction of tumor necrosis factor-alpha production by mast cells via Fc gamma R. Role of the Fc gamma RIII gamma subunit. J. Immunol. 149, 2155–2162 (1992).
De Andres, B. et al. Phosphoinositide breakdown is associated with Fc-gamma RII-mediated activation of 5′-lipoxygenase in murine eosinophils. J. Immunol. 146, 1566–1570 (1991).
Timms, J.F. et al. Identification of major binding proteins and substrates for the SH2-containing protein tyrosine phosphatase SHP-1 in macrophages. Mol. Cell. Biol. 18, 3838–3850 (1998).
Boruchov, A.M. et al. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J. Clin. Invest. 115, 2914–2923 (2005).
Song, S., Crow, A.R., Siragam, V., Freedman, J. & Lazarus, A.H. Monoclonal antibodies that mimic the action of anti-D in the amelioration of murine ITP act by a mechanism distinct from that of IVIg. Blood 105, 1546–1548 (2005).
Crow, A.R., Song, S., Semple, J.W., Freedman, J. & Lazarus, A.H. IVIg inhibits reticuloendothelial system function and ameliorates murine passive-immune thrombocytopenia independent of anti-idiotype reactivity. Br. J. Haematol. 115, 679–686 (2001).
Braun-Moscovici, Y. & Furst, D.E. Immunoglobulin for rheumatic diseases in the twenty-first century: take it or leave it? Curr. Opin. Rheumatol. 15, 237–245 (2003).
Arnal, C. et al. Treatment of severe immune thrombocytopenia associated with systemic lupus erythematosus: 59 cases. J. Rheumatol. 29, 75–83 (2002).
Spisek, R., Gasova, Z. & Bartunkova, J. Maturation state of dendritic cells during the extracorporeal photopheresis and its relevance for the treatment of chronic graft-versus-host disease. Transfusion 46, 55–65 (2006).
Song, S., Crow, A.R., Freedman, J. & Lazarus, A.H. Monoclonal IgG can ameliorate immune thrombocytopenia in a murine model of ITP: an alternative to IVIG. Blood 101, 3708–3713 (2003).
Acknowledgements
We thank J. Semple for discussion and critical review of this manuscript, H. Le-Tien and A. Starkey for assistance and discussion, and the St. Michael's Hospital Research Vivarium staff. This work was supported by grants from the Canadian Institutes of Health Research (to A.L.), and The Canadian Blood Services-Canadian Institutes of Health Research Request for Proposals Program Fund (to A.L.). V. Siragam was the recipient of a Post Doctoral Fellowship Award from the Canadian Blood Services. D. Brinc was supported by Graduate Fellowship Award from the Canadian Blood Services.
Author information
Authors and Affiliations
Contributions
V.K.S. and A.R.C. helped conceptualize and design the study, co-wrote the manuscript, performed the research and analyzed the data. D.B. and S.S. helped conceptualize and design the study and performed the research. J.F. helped conceptualize the study and co-wrote the manuscript. A.H.L. conceptualized and designed the study, obtained grant support and co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
IVIg primed B cells and T cells do not inhibit mouse ITP. (PDF 818 kb)
Supplementary Fig. 2
The requirement for IVIg can be bypassed using soluble immune complexes. (PDF 1208 kb)
Supplementary Fig. 3
IVIg does not reverse thrombocytopenia in SLE-ITP mice. (PDF 992 kb)
Rights and permissions
About this article
Cite this article
Siragam, V., Crow, A., Brinc, D. et al. Intravenous immunoglobulin ameliorates ITP via activating Fcγ receptors on dendritic cells. Nat Med 12, 688–692 (2006). https://doi.org/10.1038/nm1416
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1416
This article is cited by
-
Production of recombinant human IgG1 Fc with beneficial N-glycosylation pattern for anti-inflammatory activity using genome-edited chickens
Communications Biology (2023)
-
Intravenous immunoglobulin mediates anti-inflammatory effects in peripheral blood mononuclear cells by inducing autophagy
Cell Death & Disease (2020)
-
Using the K/BxN mouse model of endogenous, chronic, rheumatoid arthritis for the evaluation of potential immunoglobulin-based therapeutic agents, including IVIg and Fc-μTP-L309C, a recombinant IgG1 Fc hexamer
BMC Immunology (2019)
-
Potential importance of B cells in aging and aging-associated neurodegenerative diseases
Seminars in Immunopathology (2017)
-
Intravenous immune globulin suppresses angiogenesis in mice and humans
Signal Transduction and Targeted Therapy (2016)