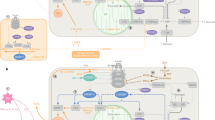
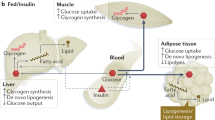
Abstract
Nutritional excess is a major forerunner of type 2 diabetes. It enhances the secretion of insulin, but attenuates insulin's metabolic actions in the liver, skeletal muscle and adipose tissue. However, conflicting evidence indicates a lack of knowledge of the timing of these events during the development of obesity and diabetes, pointing to a key gap in our understanding of metabolic disease. This Perspective reviews alternate viewpoints and recent results on the temporal and mechanistic connections between hyperinsulinemia, obesity and insulin resistance. Although much attention has addressed early steps in the insulin signaling cascade, insulin resistance in obesity seems to be largely elicited downstream of these steps. New findings also connect insulin resistance to extensive metabolic cross-talk between the liver, adipose tissue, pancreas and skeletal muscle. These and other advances over the past 5 years offer exciting opportunities and daunting challenges for the development of new therapeutic strategies for the treatment of type 2 diabetes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Reaven, G.M. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu. Rev. Nutr. 25, 391–406 (2005).
US Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2014 (CDC, 2014); available at https://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf.
Klöting, N. et al. Insulin-sensitive obesity. Am. J. Physiol. Endocrinol. Metab. 299, E506–E515 (2010).
DeFronzo, R.A., Bonadonna, R.C. & Ferrannini, E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care 15, 318–368 (1992).
Kim, S.H. & Reaven, G.M. Insulin resistance and hyperinsulinemia: you can't have one without the other. Diabetes Care 31, 1433–1438 (2008).
McGarry, J.D. What if Minkowski had been ageusic? An alternative angle on diabetes. Science 258, 766–770 (1992).
Shanik, M.H. et al. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care 31 (Suppl. 2), S262–S268 (2008).
Pories, W.J. & Dohm, G.L. Diabetes: have we got it all wrong? Hyperinsulinism as the culprit: surgery provides the evidence. Diabetes Care 35, 2438–2442 (2012).
Corkey, B.E. Banting lecture 2011: hyperinsulinemia: cause or consequence? Diabetes 61, 4–13 (2012).
Boucher, J., Kleinridders, A. & Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 6, a009191 (2014).
Parker, V.E., Savage, D.B., O'Rahilly, S. & Semple, R.K. Mechanistic insights into insulin resistance in the genetic era. Diabet. Med. 28, 1476–1486 (2011).
Klotz, L.O. et al. Redox regulation of FoxO transcription factors. Redox Biol. 6, 51–72 (2015).
Ryder, J.W., Gilbert, M. & Zierath, J.R. Skeletal muscle and insulin sensitivity: pathophysiological alterations. Front. Biosci. 6, d154–d163 (2001).
Pendergrass, M. et al. Muscle glucose transport and phosphorylation in type 2 diabetic, obese nondiabetic, and genetically predisposed individuals. Am. J. Physiol. Endocrinol. Metab. 292, E92–E100 (2007).
Nakae, J., Barr, V. & Accili, D. Differential regulation of gene expression by insulin and IGF-1 receptors correlates with phosphorylation of a single amino acid residue in the forkhead transcription factor FKHR. EMBO J. 19, 989–996 (2000).
Gross, D.N., van den Heuvel, A.P. & Birnbaum, M.J. The role of FoxO in the regulation of metabolism. Oncogene 27, 2320–2336 (2008).
Titchenell, P.M. et al. Direct hepatocyte insulin signaling is required for lipogenesis but is dispensable for the suppression of glucose production. Cell Metab. 23, 1154–1166 (2016).
Qu, S. et al. Aberrant Forkhead box O1 function is associated with impaired hepatic metabolism. Endocrinology 147, 5641–5652 (2006).
Ozcan, L. et al. Calcium signaling through CaMKII regulates hepatic glucose production in fasting and obesity. Cell Metab. 15, 739–751 (2012).
Banks, A.S. et al. Dissociation of the glucose and lipid regulatory functions of FoxO1 by targeted knockin of acetylation-defective alleles in mice. Cell Metab. 14, 587–597 (2011).
Zhang, W. et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J. Biol. Chem. 281, 10105–10117 (2006).
Perry, R.J. et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758 (2015).
Cherrington, A.D., Edgerton, D. & Sindelar, D.K. The direct and indirect effects of insulin on hepatic glucose production in vivo. Diabetologia 41, 987–996 (1998).
Rebrin, K., Steil, G.M., Mittelman, S.D. & Bergman, R.N. Causal linkage between insulin suppression of lipolysis and suppression of liver glucose output in dogs. J. Clin. Invest. 98, 741–749 (1996).
Schoiswohl, G. et al. Impact of reduced ATGL-mediated adipocyte lipolysis on obesity-associated insulin resistance and inflammation in male mice. Endocrinology 156, 3610–3624 (2015).
Kimmel, A.R. & Sztalryd, C. The perilipins: major cytosolic lipid droplet-associated proteins and their roles in cellular lipid storage, mobilization, and systemic homeostasis. Annu. Rev. Nutr. 36, 471–509 (2016).
Puri, V. et al. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc. Natl. Acad. Sci. USA 105, 7833–7838 (2008).
Gandotra, S. et al. Perilipin deficiency and autosomal dominant partial lipodystrophy. N. Engl. J. Med. 364, 740–748 (2011).
Rubio-Cabezas, O. et al. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol. Med. 1, 280–287 (2009).
Zhou, L. et al. Insulin resistance and white adipose tissue inflammation are uncoupled in energetically challenged Fsp27-deficient mice. Nat. Commun. 6, 5949 (2015).
Lotta, L.A. et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat. Genet. 49, 17–26 (2017).
Tran, T.T., Yamamoto, Y., Gesta, S. & Kahn, C.R. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 7, 410–420 (2008).
Min, S.Y. et al. Human 'brite/beige' adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat. Med. 22, 312–318 (2016).
Biddinger, S.B. et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 7, 125–134 (2008).
Brown, M.S. & Goldstein, J.L. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 7, 95–96 (2008).
Caron, A., Richard, D. & Laplante, M. The roles of mTOR complexes in lipid metabolism. Annu. Rev. Nutr. 35, 321–348 (2015).
Bar-Peled, L. & Sabatini, D.M. Regulation of mTORC1 by amino acids. Trends Cell Biol. 24, 400–406 (2014).
Beck-Nielsen, H. The role of glycogen synthase in the development of hyperglycemia in type 2 diabetes: 'To store or not to store glucose, that's the question'. Diabetes Metab. Res. Rev. 28, 635–644 (2012).
Beck-Nielsen, H., Henriksen, J.E., Vaag, A. & Hother-Nielsen, O. Pathophysiology of non-insulin-dependent diabetes mellitus (NIDDM). Diabetes Res. Clin. Pract. 28 (Suppl. ), S13–S25 (1995).
Nolan, C.J., Ruderman, N.B., Kahn, S.E., Pedersen, O. & Prentki, M. Insulin resistance as a physiological defense against metabolic stress: implications for the management of subsets of type 2 diabetes. Diabetes 64, 673–686 (2015).
Corkey, B.E. Diabetes: have we got it all wrong? Insulin hypersecretion and food additives: cause of obesity and diabetes? Diabetes Care 35, 2432–2437 (2012).
Erion, K.A., Berdan, C.A., Burritt, N.E., Corkey, B.E. & Deeney, J.T. Chronic exposure to excess nutrients left-shifts the concentration dependence of glucose-stimulated insulin secretion in pancreatic β-cells. J. Biol. Chem. 290, 16191–16201 (2015).
Kim, M.K., Reaven, G.M., Chen, Y.D., Kim, E. & Kim, S.H. Hyperinsulinemia in individuals with obesity: Role of insulin clearance. Obesity (Silver Spring) 23, 2430–2434 (2015).
Kobayashi, M. & Olefsky, J.M. Effect of experimental hyperinsulinemia on insulin binding and glucose transport in isolated rat adipocytes. Am. J. Physiol. 235, E53–E62 (1978).
Soop, M. et al. Euglycemic hyperinsulinemia augments the cytokine and endocrine responses to endotoxin in humans. Am. J. Physiol. Endocrinol. Metab. 282, E1276–E1285 (2002).
Siklova-Vitkova, M. et al. Effect of hyperinsulinemia and very-low-calorie diet on interstitial cytokine levels in subcutaneous adipose tissue of obese women. Am. J. Physiol. Endocrinol. Metab. 297, E1154–E1161 (2009).
Murdolo, G. et al. Monocyte chemoattractant protein-1 in subcutaneous abdominal adipose tissue: characterization of interstitial concentration and regulation of gene expression by insulin. J. Clin. Endocrinol. Metab. 92, 2688–2695 (2007).
Westerbacka, J. et al. Acute in vivo effects of insulin on gene expression in adipose tissue in insulin-resistant and insulin-sensitive subjects. Diabetologia 49, 132–140 (2006).
Westerbacka, J. et al. Insulin regulation of MCP-1 in human adipose tissue of obese and lean women. Am. J. Physiol. Endocrinol. Metab. 294, E841–E845 (2008).
Krogh-Madsen, R., Plomgaard, P., Keller, P., Keller, C. & Pedersen, B.K. Insulin stimulates interleukin-6 and tumor necrosis factor-alpha gene expression in human subcutaneous adipose tissue. Am. J. Physiol. Endocrinol. Metab. 286, E234–E238 (2004).
Jansen, H.J. et al. Start of insulin therapy in patients with type 2 diabetes mellitus promotes the influx of macrophages into subcutaneous adipose tissue. Diabetologia 56, 2573–2581 (2013).
Lackey, D.E. & Olefsky, J.M. Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 12, 15–28 (2016).
Tsiotra, P.C., Boutati, E., Dimitriadis, G. & Raptis, S.A. High insulin and leptin increase resistin and inflammatory cytokine production from human mononuclear cells. BioMed Res. Int. 2013, 487081 (2013).
Pedersen, D.J. et al. A major role of insulin in promoting obesity-associated adipose tissue inflammation. Mol. Metab. 4, 507–518 (2015).
Roth Flach, R.J. et al. Protein kinase mitogen-activated protein kinase kinase kinase kinase 4 (MAP4K4) promotes obesity-induced hyperinsulinemia. J. Biol. Chem. 291, 16221–16230 (2016).
Boden, G., Chen, X., Rosner, J. & Barton, M. Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes 44, 1239–1242 (1995).
Stein, D.T. et al. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J. Clin. Invest. 97, 2728–2735 (1996).
Deeney, J.T. et al. Acute stimulation with long chain acyl-CoA enhances exocytosis in insulin-secreting cells (HIT T-15 and NMRI beta-cells). J. Biol. Chem. 275, 9363–9368 (2000).
Templeman, N.M., Clee, S.M. & Johnson, J.D. Suppression of hyperinsulinaemia in growing female mice provides long-term protection against obesity. Diabetologia 58, 2392–2402 (2015).
Mehran, A.E. et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 16, 723–737 (2012).
D'souza, A.M., Johnson, J.D., Clee, S.M. & Kieffer, T.J. Suppressing hyperinsulinemia prevents obesity but causes rapid onset of diabetes in leptin-deficient Lep(ob/ob) mice. Mol. Metab. 5, 1103–1112 (2016).
Nedergaard, J. & Cannon, B. The browning of white adipose tissue: some burning issues. Cell Metab. 20, 396–407 (2014).
Liu, D. et al. Activation of mTORC1 is essential for β-adrenergic stimulation of adipose browning. J. Clin. Invest. 126, 1704–1716 (2016).
Marban, S.L.R.J. Transgenic Hyperinsulinemia: A Mouse Model of Insulin Resistance and Glucose Intolerance without Obesity (Shafrir E, Boston, Birkhauser, 1996).
Pontiroli, A.E., Alberetto, M., Capra, F. & Pozza, G. The glucose clamp technique for the study of patients with hypoglycemia: insulin resistance as a feature of insulinoma. J. Endocrinol. Invest. 13, 241–245 (1990).
Manousaki, D. et al. Toward precision medicine: TBC1D4 disruption is common among the inuit and leads to underdiagnosis of type 2 diabetes. Diabetes Care 39, 1889–1895 (2016).
Chen, D.L. et al. Phenotypic characterization of insulin-resistant and insulin-sensitive obesity. J. Clin. Endocrinol. Metab. 100, 4082–4091 (2015).
Waise, T.M. et al. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochem. Biophys. Res. Commun. 464, 1157–1162 (2015).
Turner, N. et al. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 56, 1638–1648 (2013).
Scherer, T. et al. Short term voluntary overfeeding disrupts brain insulin control of adipose tissue lipolysis. J. Biol. Chem. 287, 33061–33069 (2012).
Paglialunga, S., Ludzki, A., Root-McCaig, J. & Holloway, G.P. In adipose tissue, increased mitochondrial emission of reactive oxygen species is important for short-term high-fat diet-induced insulin resistance in mice. Diabetologia 58, 1071–1080 (2015).
Barzel, B. et al. Short term fat feeding rapidly increases plasma insulin but does not result in dyslipidaemia. Front. Physiol. 5, 469 (2014).
Ji, Y. et al. Short term high fat diet challenge promotes alternative macrophage polarization in adipose tissue via natural killer T cells and interleukin-4. J. Biol. Chem. 287, 24378–24386 (2012).
Lee, Y.S. et al. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 60, 2474–2483 (2011).
Ben-Shlomo, S. et al. Perinephric and epididymal fat affect hepatic metabolism in rats. Obesity (Silver Spring) 20, 151–156 (2012).
Commerford, S.R. et al. Diets enriched in sucrose or fat increase gluconeogenesis and G-6-Pase but not basal glucose production in rats. Am. J. Physiol. Endocrinol. Metab. 283, E545–E555 (2002).
Boden, G. et al. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Sci. Transl. Med. 7, 304re7 (2015).
Brøns, C. et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J. Physiol. (Lond.) 587, 2387–2397 (2009).
Lagerpusch, M., Bosy-Westphal, A., Kehden, B., Peters, A. & Müller, M.J. Effects of brief perturbations in energy balance on indices of glucose homeostasis in healthy lean men. Int. J. Obes. 36, 1094–1101 (2012).
Olefsky, J., Crapo, P.A., Ginsberg, H. & Reaven, G.M. Metabolic effects of increased caloric intake in man. Metabolism 24, 495–503 (1975).
Wadden, D. et al. Serum acylated ghrelin concentrations in response to short-term overfeeding in normal weight, overweight, and obese men. PLoS One 7, e45748 (2012).
Cahill, F., Shea, J.L., Randell, E., Vasdev, S. & Sun, G. Serum peptide YY in response to short-term overfeeding in young men. Am. J. Clin. Nutr. 93, 741–747 (2011).
Numao, S. et al. Effects of a single bout of aerobic exercise on short-term low-carbohydrate/high-fat intake-induced postprandial glucose metabolism during an oral glucose tolerance test. Metabolism 62, 1406–1415 (2013).
Drucker, D.J. Deciphering metabolic messages from the gut drives therapeutic innovation: the 2014 Banting Lecture. Diabetes 64, 317–326 (2015).
Perry, R.J. et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 534, 213–217 (2016).
Lo, J.C. et al. Adipsin is an adipokine that improves β cell function in diabetes. Cell 158, 41–53 (2014).
Kraegen, E.W. et al. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes 40, 1397–1403 (1991).
Abdul-Ghani, M.A., Jenkinson, C.P., Richardson, D.K., Tripathy, D. & DeFronzo, R.A. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 55, 1430–1435 (2006).
Samuel, V.T. & Shulman, G.I. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J. Clin. Invest. 126, 12–22 (2016).
Copps, K.D. & White, M.F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55, 2565–2582 (2012).
Petersen, M.C. et al. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J. Clin. Invest. 126, 4361–4371 (2016).
Chaurasia, B. & Summers, S.A. Ceramides – Lipotoxic Inducers of Metabolic Disorders. Trends Endocrinol. Metab. 26, 538–550 (2015).
Zhang, C. et al. Inhibited insulin signaling in mouse hepatocytes is associated with increased phosphatidic acid but not diacylglycerol. J. Biol. Chem. 290, 3519–3528 (2015).
Guilherme, A., Virbasius, J.V., Puri, V. & Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9, 367–377 (2008).
Kang, S., Tsai, L.T. & Rosen, E.D. Nuclear mechanisms of insulin resistance. Trends Cell Biol. 26, 341–351 (2016).
Kusminski, C.M., Bickel, P.E. & Scherer, P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug Discov. 15, 639–660 (2016).
Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 15, 606–614 (2012).
Sabio, G. & Davis, R.J. cJun NH2-terminal kinase 1 (JNK1): roles in metabolic regulation of insulin resistance. Trends Biochem. Sci. 35, 490–496 (2010).
Hoehn, K.L. et al. IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 7, 421–433 (2008).
Mîinea, C.P. et al. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem. J. 391, 87–93 (2005).
Sun, K. et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat. Commun. 5, 3485 (2014).
Barrett, E.J., Wang, H., Upchurch, C.T. & Liu, Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am. J. Physiol. Endocrinol. Metab. 301, E252–E263 (2011).
Lee, W.L. & Klip, A. Endothelial transcytosis of insulin: does it contribute to insulin resistance? Physiology (Bethesda) 31, 336–345 (2016).
Williams, A.S., Kang, L. & Wasserman, D.H. The extracellular matrix and insulin resistance. Trends Endocrinol. Metab. 26, 357–366 (2015).
Williams, A.S. et al. Integrin α1-null mice exhibit improved fatty liver when fed a high fat diet despite severe hepatic insulin resistance. J. Biol. Chem. 290, 6546–6557 (2015).
Kang, L. et al. Diet-induced muscle insulin resistance is associated with extracellular matrix remodeling and interaction with integrin α2β1 in mice. Diabetes 60, 416–426 (2011).
Kang, L. et al. Integrin-linked kinase in muscle is necessary for the development of insulin resistance in diet-induced obese mice. Diabetes 65, 1590–1600 (2016).
Abdennour, M. et al. Association of adipose tissue and liver fibrosis with tissue stiffness in morbid obesity: links with diabetes and BMI loss after gastric bypass. J. Clin. Endocrinol. Metab. 99, 898–907 (2014).
Corvera, S. & Gealekman, O. Adipose tissue angiogenesis: impact on obesity and type-2 diabetes. Biochim. Biophys. Acta 1842, 463–472 (2014).
Gealekman, O. et al. Control of adipose tissue expandability in response to high fat diet by the insulin-like growth factor-binding protein-4. J. Biol. Chem. 289, 18327–18338 (2014).
Furler, S.M., Jenkins, A.B., Storlien, L.H. & Kraegen, E.W. In vivo location of the rate-limiting step of hexose uptake in muscle and brain tissue of rats. Am. J. Physiol. 261, E337–E347 (1991).
Zierath, J.R., Krook, A. & Wallberg-Henriksson, H. Insulin action in skeletal muscle from patients with NIDDM. Mol. Cell. Biochem. 182, 153–160 (1998).
Turner, N., Cooney, G.J., Kraegen, E.W. & Bruce, C.R. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J. Endocrinol. 220, T61–T79 (2014).
Bonadonna, R.C. et al. Roles of glucose transport and glucose phosphorylation in muscle insulin resistance of NIDDM. Diabetes 45, 915–925 (1996).
Cline, G.W. et al. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N. Engl. J. Med. 341, 240–246 (1999).
Petersen, K.F. & Shulman, G.I. Cellular mechanism of insulin resistance in skeletal muscle. J. R. Soc. Med. 95 (Suppl. 42), 8–13 (2002).
Kelley, D.E. et al. The effect of non-insulin-dependent diabetes mellitus and obesity on glucose transport and phosphorylation in skeletal muscle. J. Clin. Invest. 97, 2705–2713 (1996).
Williams, K.V., Price, J.C. & Kelley, D.E. Interactions of impaired glucose transport and phosphorylation in skeletal muscle insulin resistance: a dose-response assessment using positron emission tomography. Diabetes 50, 2069–2079 (2001).
Perry, R.J., Zhang, D., Zhang, X.M., Boyer, J.L. & Shulman, G.I. Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science 347, 1253–1256 (2015).
Montgomery, M.K. & Turner, N. Mitochondrial dysfunction and insulin resistance: an update. Endocr. Connect. 4, R1–R15 (2015).
Patti, M.E. & Corvera, S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr. Rev. 31, 364–395 (2010).
Czech, M.P. Cellular basis of insulin insensitivity in large rat adipocytes. J. Clin. Invest. 57, 1523–1532 (1976).
Kahn, B.B. Lilly lecture 1995. Glucose transport: pivotal step in insulin action. Diabetes 45, 1644–1654 (1996).
Gonzalez, E., Flier, E., Molle, D., Accili, D. & McGraw, T.E. Hyperinsulinemia leads to uncoupled insulin regulation of the GLUT4 glucose transporter and the FoxO1 transcription factor. Proc. Natl. Acad. Sci. USA 108, 10162–10167 (2011).
Sabio, G. et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 322, 1539–1543 (2008).
Tan, S.X. et al. Amplification and demultiplexing in insulin-regulated Akt protein kinase pathway in adipocytes. J. Biol. Chem. 287, 6128–6138 (2012).
Tan, S.X. et al. Selective insulin resistance in adipocytes. J. Biol. Chem. 290, 11337–11348 (2015).
Morley, T.S., Xia, J.Y. & Scherer, P.E. Selective enhancement of insulin sensitivity in the mature adipocyte is sufficient for systemic metabolic improvements. Nat. Commun. 6, 7906 (2015).
Thomson, M.J., Williams, M.G. & Frost, S.C. Development of insulin resistance in 3T3-L1 adipocytes. J. Biol. Chem. 272, 7759–7764 (1997).
Richardson, D.K. & Czech, M.P. Primary role of decreased fatty acid synthesis in insulin resistance of large rat adipocytes. Am. J. Physiol. 234, E182–E189 (1978).
Czech, M.P., Tencerova, M., Pedersen, D.J. & Aouadi, M. Insulin signalling mechanisms for triacylglycerol storage. Diabetologia 56, 949–964 (2013).
Herman, M.A. et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 484, 333–338 (2012).
Tang, Y. et al. Adipose tissue mTORC2 regulates ChREBP-driven de novo lipogenesis and hepatic glucose metabolism. Nat. Commun. 7, 11365 (2016).
Solinas, G., Borén, J. & Dulloo, A.G. De novo lipogenesis in metabolic homeostasis: More friend than foe? Mol. Metab. 4, 367–377 (2015).
Baraille, F., Planchais, J., Dentin, R., Guilmeau, S. & Postic, C. Integration of ChREBP-mediated glucose sensing into whole body metabolism. Physiology (Bethesda) 30, 428–437 (2015).
Lodhi, I.J. et al. Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARγ activation to decrease diet-induced obesity. Cell Metab. 16, 189–201 (2012).
Yore, M.M. et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 159, 318–332 (2014).
Smith, U. & Kahn, B.B. Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. J. Intern. Med. 280, 465–475 (2016).
Guilherme, A. et al. Adipocyte lipid synthesis coupled to neuronal control of thermogenic programming. Mol. Metab. (in the press).
Dutta, A., Abmayr, S.M. & Workman, J.L. Diverse activities of histone acylations connect metabolism to chromatin function. Mol. Cell 63, 547–552 (2016).
Wellen, K.E. & Thompson, C.B. A two-way street: reciprocal regulation of metabolism and signalling. Nat. Rev. Mol. Cell Biol. 13, 270–276 (2012).
Londoño Gentile, T. et al. DNMT1 is regulated by ATP-citrate lyase and maintains methylation patterns during adipocyte differentiation. Mol. Cell. Biol. 33, 3864–3878 (2013).
Wellen, K.E. et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080 (2009).
McDonnell, E. et al. Lipids reprogram metabolism to become a major carbon source for histone acetylation. Cell Rep. 17, 1463–1472 (2016).
Lefterova, M.I., Haakonsson, A.K., Lazar, M.A. & Mandrup, S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 25, 293–302 (2014).
Sugii, S. & Evans, R.M. Epigenetic codes of PPARγ in metabolic disease. FEBS Lett. 585, 2121–2128 (2011).
Wilson-Fritch, L. et al. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J. Clin. Invest. 114, 1281–1289 (2004).
Choi, J.H. et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature 466, 451–456 (2010).
Soccio, R.E. et al. Genetic variation determines PPARγ function and anti-diabetic drug response in vivo. Cell 162, 33–44 (2015).
Soccio, R.E. et al. Targeting PPARγ in the epigenome rescues genetic metabolic defects in mice. J. Clin. Invest. 127, 1451–1462 (2017).
Kienesberger, P.C. et al. Adipose triglyceride lipase deficiency causes tissue-specific changes in insulin signaling. J. Biol. Chem. 284, 30218–30229 (2009).
Zechner, R. FAT FLUX: enzymes, regulators, and pathophysiology of intracellular lipolysis. EMBO Mol. Med. 7, 359–362 (2015).
Kory, N., Farese, R.V. Jr. & Walther, T.C. Targeting fat: mechanisms of protein localization to lipid droplets. Trends Cell Biol. 26, 535–546 (2016).
Krahmer, N., Farese, R.V. Jr. & Walther, T.C. Balancing the fat: lipid droplets and human disease. EMBO Mol. Med. 5, 973–983 (2013).
Degerman, E. et al. Phosphorylation and activation of hormone-sensitive adipocyte phosphodiesterase type 3B. Methods 14, 43–53 (1998).
Choi, Y.H. et al. Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B-null mice. J. Clin. Invest. 116, 3240–3251 (2006).
Kitamura, T. et al. Insulin-induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serine-threonine kinase Akt. Mol. Cell. Biol. 19, 6286–6296 (1999).
Choi, S.M. et al. Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Mol. Cell. Biol. 30, 5009–5020 (2010).
Koren, S. et al. The role of mouse Akt2 in insulin-dependent suppression of adipocyte lipolysis in vivo. Diabetologia 58, 1063–1070 (2015).
DiPilato, L.M. et al. The role of PDE3B phosphorylation in the inhibition of lipolysis by insulin. Mol. Cell. Biol. 35, 2752–2760 (2015).
Shin, A.C. et al. Insulin receptor signaling in POMC, but not AgRP, neurons controls adipose tissue insulin action. Diabetes 66, 1560–1571 (2017).
Vigneri, R., Goldfine, I.D. & Frittitta, L. Insulin, insulin receptors, and cancer. J. Endocrinol. Invest. 39, 1365–1376 (2016).
Zierler, K.A. et al. Functional cardiac lipolysis in mice critically depends on comparative gene identification-58. J. Biol. Chem. 288, 9892–9904 (2013).
Whiteman, E.L., Cho, H. & Birnbaum, M.J. Role of Akt/protein kinase B in metabolism. Trends Endocrinol. Metab. 13, 444–451 (2002).
Cho, H. et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292, 1728–1731 (2001).
Jiang, Z.Y. et al. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc. Natl. Acad. Sci. USA 100, 7569–7574 (2003).
Leavens, K.F., Easton, R.M., Shulman, G.I., Previs, S.F. & Birnbaum, M.J. Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 10, 405–418 (2009).
Acknowledgements
I thank M. Birnbaum (Pfizer), S. O'Rahilly (University of Cambridge), and S. Corvera, J. Virbasius, A. Guilherme and D. Pedersen (University of Massachusetts Medical School) for their critical reading of the manuscript and their helpful comments. I also thank our laboratory group members for stimulating discussions on these topics, and L. Smith (University of Massachusetts Medical School) for her contributions to the formatting and editing of the manuscript. The work cited from our laboratory was funded by US National Institutes of Health (NIH) grants DK 103047 and DK 030898, and the Isadore and Fannie Foxman endowed professorship in medical science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Czech, M. Insulin action and resistance in obesity and type 2 diabetes. Nat Med 23, 804–814 (2017). https://doi.org/10.1038/nm.4350
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4350
This article is cited by
-
Relationship between TyG index and the degree of coronary artery lesions in patients with H-type hypertension
Cardiovascular Diabetology (2024)
-
Hepatic glucose metabolism in the steatotic liver
Nature Reviews Gastroenterology & Hepatology (2024)
-
Gastric Submucosal Fat Accumulation Is Associated with Insulin Resistance in Patients with Obesity
Obesity Surgery (2024)
-
STING signaling in islet macrophages impairs insulin secretion in obesity
Science China Life Sciences (2024)
-
Insulin Resistance/Diabetes and Schizophrenia: Potential Shared Genetic Factors and Implications for Better Management of Patients with Schizophrenia
CNS Drugs (2024)