Abstract
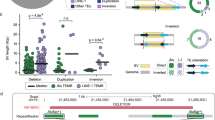
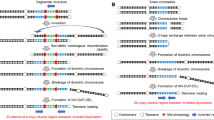
We identified complex genomic rearrangements consisting of intermixed duplications and triplications of genomic segments at the MECP2 and PLP1 loci. These complex rearrangements were characterized by a triplicated segment embedded within a duplication in 11 unrelated subjects. Notably, only two breakpoint junctions were generated during each rearrangement formation. All the complex rearrangement products share a common genomic organization, duplication-inverted triplication-duplication (DUP-TRP/INV-DUP), in which the triplicated segment is inverted and located between directly oriented duplicated genomic segments. We provide evidence that the DUP-TRP/INV-DUP structures are mediated by inverted repeats that can be separated by >300 kb, a genomic architecture that apparently leads to susceptibility to such complex rearrangements. A similar inverted repeat–mediated mechanism may underlie structural variation in many other regions of the human genome. We propose a mechanism that involves both homology-driven events, via inverted repeats, and microhomologous or nonhomologous events.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Carvalho, C.M. et al. Complex rearrangements in patients with duplications of MECP2 can occur by fork stalling and template switching. Hum. Mol. Genet. 18, 2188–2203 (2009).
Morrow, D.M., Connelly, C. & Hieter, P. “Break copy” duplication: a model for chromosome fragment formation in Saccharomyces cerevisiae. Genetics 147, 371–382 (1997).
Smith, C.E., Llorente, B. & Symington, L.S. Template switching during break-induced replication. Nature 447, 102–105 (2007).
McEachern, M.J. & Haber, J.E. Break-induced replication and recombinational telomere elongation in yeast. Annu. Rev. Biochem. 75, 111–135 (2006).
Hastings, P.J., Ira, G. & Lupski, J.R. A microhomology-mediated break-induced replication model for the origin of human copy number variation. PLoS Genet. 5, e1000327 (2009).
Hastings, P.J., Lupski, J.R., Rosenberg, S.M. & Ira, G. Mechanisms of change in gene copy number. Nat. Rev. Genet. 10, 551–564 (2009).
Lee, J.A., Carvalho, C.M. & Lupski, J.R. A DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell 131, 1235–1247 (2007).
Slack, A., Thornton, P.C., Magner, D.B., Rosenberg, S.M. & Hastings, P.J. On the mechanism of gene amplification induced under stress in Escherichia coli. PLoS Genet. 2, e48 (2006).
del Gaudio, D. et al. Increased MECP2 gene copy number as the result of genomic duplication in neurodevelopmentally delayed males. Genet. Med. 8, 784–792 (2006).
Small, K., Iber, J. & Warren, S.T. Emerin deletion reveals a common X-chromosome inversion mediated by inverted repeats. Nat. Genet. 16, 96–99 (1997).
Deem, A. et al. Break-induced replication is highly inaccurate. PLoS Biol. 9, e1000594 (2011).
Lee, J.A. et al. Spastic paraplegia type 2 associated with axonal neuropathy and apparent PLP1 position effect. Ann. Neurol. 59, 398–403 (2006).
Ramocki, M.B., Tavyev, Y.J. & Peters, S.U. The MECP2 duplication syndrome. Am. J. Med. Genet. A. 152A, 1079–1088 (2010).
Bailey, J.A. et al. Recent segmental duplications in the human genome. Science 297, 1003–1007 (2002).
Hu, X.Y., Ray, P.N., Murphy, E.G., Thompson, M.W. & Worton, R.G. Duplicational mutation at the Duchenne muscular dystrophy locus: its frequency, distribution, origin, and phenotype-genotype correlation. Am. J. Hum. Genet. 46, 682–695 (1990).
Mimault, C. et al. Proteolipoprotein gene analysis in 82 patients with sporadic Pelizaeus-Merzbacher disease: duplications, the major cause of the disease, originate more frequently in male germ cells, but point mutations do not. The Clinical European Network on Brain Dysmyelinating Disease. Am. J. Hum. Genet. 65, 360–369 (1999).
Whibley, A.C. et al. Fine-scale survey of X chromosome copy number variants and indels underlying intellectual disability. Am. J. Hum. Genet. 87, 173–188 (2010).
Bartsch, O. et al. Four unrelated patients with Lubs X-linked mental retardation syndrome and different Xq28 duplications. Am. J. Med. Genet. A. 152A, 305–312 (2010).
Lieber, M.R. The mechanism of human nonhomologous DNA end joining. J. Biol. Chem. 283, 1–5 (2008).
Sheen, C.R. et al. Double complex mutations involving F8 and FUNDC2 caused by distinct break-induced replication. Hum. Mutat. 28, 1198–1206 (2007).
Zhang, F., Carvalho, C.M. & Lupski, J.R. Complex human chromosomal and genomic rearrangements. Trends Genet. 25, 298–307 (2009).
Zhang, F. et al. The DNA replication FoSTeS/MMBIR mechanism can generate genomic, genic and exonic complex rearrangements in humans. Nat. Genet. 41, 849–853 (2009).
Yatsenko, S.A. et al. Molecular mechanisms for subtelomeric rearrangements associated with the 9q34.3 microdeletion syndrome. Hum. Mol. Genet. 18, 1924–1936 (2009).
Vialard, F. et al. Mechanism of intrachromosomal triplications 15q11-q13: a new clinical report. Am. J. Med. Genet. A. 118A, 229–234 (2003).
Wang, J. et al. Intrachromosomal triplication of 2q11.2-q21 in a severely malformed infant: case report and review of triplications and their possible mechanism. Am. J. Med. Genet. 82, 312–317 (1999).
Reddy, K.S. & Logan, J.J. Intrachromosomal triplications: molecular cytogenetic and clinical studies. Clin. Genet. 58, 134–141 (2000).
Lebofsky, R. & Bensimon, A. DNA replication origin plasticity and perturbed fork progression in human inverted repeats. Mol. Cell Biol. 25, 6789–6797 (2005).
Voineagu, I., Narayanan, V., Lobachev, K.S. & Mirkin, S.M. Replication stalling at unstable inverted repeats: interplay between DNA hairpins and fork stabilizing proteins. Proc. Natl. Acad. Sci. USA 105, 9936–9941 (2008).
Paek, A.L. et al. Fusion of nearby inverted repeats by a replication-based mechanism leads to formation of dicentric and acentric chromosomes that cause genome instability in budding yeast. Genes Dev. 23, 2861–2875 (2009).
Mizuno, K., Lambert, S., Baldacci, G., Murray, J.M. & Carr, A.M. Nearby inverted repeats fuse to generate acentric and dicentric palindromic chromosomes by a replication template exchange mechanism. Genes Dev. 23, 2876–2886 (2009).
Kitamura, E., Blow, J.J. & Tanaka, T.U. Live-cell imaging reveals replication of individual replicons in eukaryotic replication factories. Cell 125, 1297–1308 (2006).
Mirkin, E.V. & Mirkin, S.M. Mechanisms of transcription-replication collisions in bacteria. Mol. Cell Biol. 25, 888–895 (2005).
Merrikh, H., Machon, C., Grainger, W.H., Grossman, A.D. & Soultanas, P. Co-directional replication-transcription conflicts lead to replication restart. Nature 470, 554–557 (2011).
Cáceres, M., Sullivan, R.T. & Thomas, J.W. A recurrent inversion on the eutherian X chromosome. Proc. Natl. Acad. Sci. USA 104, 18571–18576 (2007).
Lee, J.A., Cheung, S.W., Ward, P.A., Inoue, K. & Lupski, J.R. Prenatal diagnosis of PLP1 copy number by array comparative genomic hybridization. Prenat. Diagn. 25, 1188–1191 (2005).
Singleton, A.B. et al. α-Synuclein locus triplication causes Parkinson's disease. Science 302, 841 (2003).
Beunders, G. et al. A triplication of the Williams-Beuren syndrome region in a patient with mental retardation, a severe expressive language delay, behavioral problems and dysmorphisms. J. Med. Genet. 47, 271–275 (2010).
Lee, J.A. et al. Role of genomic architecture in PLP1 duplication causing Pelizaeus-Merzbacher disease. Hum. Mol. Genet. 15, 2250–2265 (2006).
Allen, R.C., Zoghbi, H.Y., Moseley, A.B., Rosenblatt, H.M. & Belmont, J.W. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am. J. Hum. Genet. 51, 1229–1239 (1992).
Ramocki, M.B. et al. Autism and other neuropsychiatric symptoms are prevalent in individuals with MECP2 duplication syndrome. Ann. Neurol. 66, 771–782 (2009).
Barrett, J.C., Fry, B., Maller, J. & Daly, M.J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265 (2005).
Acknowledgements
We thank all of the families who participated in this study. We acknowledge P. Stankiewicz for helpful discussions. This work was supported in part by US National Institute of Neurological Disorders and Stroke (NINDS) grant R01 NS058529 to J.R.L., NINDS grant R01 HD053862 to H.Y.Z., US National Institute of General Medical Sciences (NIGMS) grant R01 GM064022 to P.J.H. and NINDS grant 5K08NS062711-03 to M.B.R. Lymphoblast cell lines were developed by the Baylor College of Medicine Intellectual and Developmental Disabilities Research Center cell culture core, which is funded by award P30HD024064 from the Eunice Kennedy Shriver US National Institute of Child Health and Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS, NIGMS, NICHD or the US National Institutes of Health. H.Y.Z. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
C.M.B.C. conducted high-density aCGH, FISH, breakpoint sequencing, Southern-blotting experiments and data analysis. M.B.R. coordinated human studies, recruited subjects and analyzed clinical data. D.P. and P.L. assisted with high-density aCGH and breakpoint sequencing. L.M.F. and J.W.B. conducted SNP genotyping. E.K.P., S.H.-D., L.S., L.F., S.L. and R.S. recruited and clinically characterized subjects. C.G.-J. assisted with data analysis. P.F. conducted the X-chromosome inactivation studies. A.M. and M.W. carried out cell culture. D.d.G. conducted MLPA. S.W.C. was involved in cytogenetic and clinical aCGH studies. J.R.L. and H.Y.Z. were involved in research design and data analyses. P.J.H. was involved in data analyses. C.M.B.C., M.B.R., P.J.H. and J.R.L. prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.R.L. is on the scientific advisory board of Ion Torrent Systems, is a consultant for Athena Diagnostics and has stock ownership in 23andMe. P.F., D.d.G., S.W.C. and J.R.L. are based in the Department of Molecular and Human Genetics at Baylor College of Medicine, which derives clinical income from the application of high resolution human genome analyses.
Supplementary information
Supplementary Text and Figures
Supplementary Note, Supplementary Tables 1–4 and Supplementary Figures 1–7 (PDF 441 kb)
Rights and permissions
About this article
Cite this article
Carvalho, C., Ramocki, M., Pehlivan, D. et al. Inverted genomic segments and complex triplication rearrangements are mediated by inverted repeats in the human genome. Nat Genet 43, 1074–1081 (2011). https://doi.org/10.1038/ng.944
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.944
This article is cited by
-
Complex rearrangement in TBC1D4 in an individual with diabetes due to severe insulin resistance syndrome
European Journal of Human Genetics (2024)
-
Identification and characterization of two DMD pedigrees with large inversion mutations based on a long-read sequencing pipeline
European Journal of Human Genetics (2023)
-
IRAK1 Duplication in MECP2 Duplication Syndrome Does Not Increase Canonical NF-κB–Induced Inflammation
Journal of Clinical Immunology (2023)
-
16p13.11p11.2 triplication syndrome: a new recognizable genomic disorder characterized by optical genome mapping and whole genome sequencing
European Journal of Human Genetics (2022)
-
Circular DNA intermediates in the generation of large human segmental duplications
BMC Genomics (2020)