Abstract
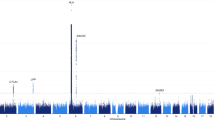
To understand the genetic predisposition to selective immunoglobulin A deficiency (IgAD), we performed a genome-wide association study in 430 affected individuals (cases) from Sweden and Iceland and 1,090 ethnically matched controls, and we performed replication studies in two independent European cohorts. In addition to the known association of HLA with IgAD, we identified association with a nonsynonymous variant in IFIH1 (rs1990760G>A, P = 7.3 × 10−10) which was previously associated with type 1 diabetes and systemic lupus erythematosus. Variants in CLEC16A, another known autoimmunity locus, showed suggestive evidence for association (rs6498142C>G, P = 1.8 × 10−7), and 29 additional loci were identified with P < 5 × 10−5. A survey in IgAD of 118 validated non-HLA autoimmunity loci indicated a significant enrichment for association with autoimmunity loci as compared to non-autoimmunity loci (P = 9.0 × 10−4) or random SNPs across the genome (P < 0.0001). These findings support the hypothesis that autoimmune mechanisms may contribute to the pathogenesis of IgAD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Casanova, J.-L. & Abel, L. Primary immunodeficiencies: a field in its infancy. Science 317, 617–619 (2007).
Notarangelo, L.D. et al. Primary immunodeficiencies: 2009 update. J. Allergy Clin. Immunol. 124, 1161–1178 (2009).
Conley, M.E., Notarangelo, L.D. & Etzioni, A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin. Immunol. 93, 190–197 (1999).
Burrows, P.D. & Cooper, M.D. IgA deficiency. Adv. Immunol. 65, 245–276 (1997).
Hammarström, L., Vorechovsky, I. & Webster, D. Selective IgA deficiency (SIgAD) and common variable immunodeficiency (CVID). Clin. Exp. Immunol. 120, 225–231 (2000).
Fagarasan, S. & Honjo, T. Intestinal IgA synthesis: regulation of front-line body defences. Nat. Rev. Immunol. 3, 63–72 (2003).
Cerutti, A. & Rescigno, M. The biology of intestinal immunoglobulin A responses. Immunity 28, 740–750 (2008).
Cunningham-Rundles, C. Physiology of IgA and IgA Deficiency. J. Clin. Immunol. 21, 303–309 (2001).
Vorechovský, I., Webster, A.D., Plebani, A. & Hammarstrom, L. Genetic linkage of IgA deficiency to the major histocompatibility complex: evidence for allele segregation distortion, parent-of-origin penetrance differences, and the role of anti-IgA antibodies in disease predisposition. Am. J. Hum. Genet. 64, 1096–1109 (1999).
Vorechovský, I. et al. Family and linkage study of selective IgA deficiency and common variable immunodeficiency. Clin. Immunol. Immunopathol. 77, 185–192 (1995).
Price, A.L. et al. Discerning the ancestry of European Americans in genetic association studies. PLoS Genet. 4, e236 (2008).
Tian, C. et al. Analysis and application of European genetic substructure using 300 K SNP information. PLoS Genet. 4, e4 (2008).
Smyth, D.J. et al. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat. Genet. 38, 617–619 (2006).
Gateva, V. et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat. Genet. 41, 1228–1233 (2009).
Kato, H. et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 (2006).
Nejentsev, S., Walker, N., Riches, D., Egholm, M. & Todd, J.A. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science 324, 387–389 (2009).
Shigemoto, T. et al. Identification of loss of function mutations in human genes encoding RIG-I and MDA5: implications for resistance to type I diabetes. J. Biol. Chem. 284, 13348–13354 (2009).
Hakonarson, H. et al. A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature 448, 591–594 (2007).
International Multiple Sclerosis Genetics Consortium (IMSGC). The expanding genetic overlap between multiple sclerosis and type I diabetes. Genes Immun. 10, 11–14 (2009).
Goshen, E. et al. Antinuclear and related autoantibodies in sera of healthy subjects with IgA deficiency. J. Autoimmun. 2, 51–60 (1989).
Barka, N. et al. Multireactive pattern of serum autoantibodies in asymptomatic individuals with immunoglobulin A deficiency. Clin. Diagn. Lab. Immunol. 2, 469–472 (1995).
Hammarström, L., Persson, M.A. & Smith, C.I. Anti-IgA in selective IgA deficiency. In vitro effects and Ig subclass pattern of human anti-IgA. Scand. J. Immunol. 18, 509–513 (1983).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Price, A.L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Salmela, E. et al. Genome-wide analysis of single nucleotide polymorphisms uncovers population structure in Northern Europe. PLoS ONE 3, e3519 (2008).
Marchini, J., Howie, B., Myers, S., McVean, G. & Donnelly, P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat. Genet. 39, 906–913 (2007).
Acknowledgements
We are grateful to the individuals with IgAD and their physicians for their contributions to this work. These studies were supported by the US National Institutes of Health (U19 AI067152 and AR043274), the Swedish Research Council, the European Research Council (242551-ImmunoSwitch) and EURO-PADnet grant 201549. Genentech, Inc. provided partial funding for the GWAS studies. Financial support was also provided through the regional agreement on medical training and clinical research the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet between the Stockholm county council and the Karolinska Institute. R.C.F. was supported by a fellowship from the Fundação para a Ciência e Tecnologia, Portugal (SFRH/BD/16281/2004). The population allele and genotype frequencies were obtained from a data source funded by the Nordic Center of Excellence in Disease Genetics based on regional samples from Finland and Sweden. The Helsinki 4 study was supported by the Yale Center for Human Genetics and Genomics and the Yale Program on Neurogenetics and US National Institutes of Health grants R01NS057756 and U24 NS051869.
Author information
Authors and Affiliations
Contributions
R.C.F. carried out most of the analyses for this study. T.W.B. and L.H. conceived and directed this study. G.F., E.U., M.F.-A., C.N., G.J., B.R.L., S.K., K.H., L.K., P.K.G. and L.H. performed subject diagnosis, coordinated the enrollment of subjects and provided access to genotyping datasets. A.T.L. performed sample genotyping in the laboratory of P.K.G. Q.P.-H., V.G., R.R.G., W.O. and H.F.C. contributed to data access and analysis. R.C.F., T.W.B. and L.H. wrote the manuscript with collaboration from Q.P.-H. and R.R.G. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
R.R.G., V.G., W.O., H.F.K. and T.W.B. are full-time employees of Genentech, Inc.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3, Supplementary Tables 1–8 and Supplementary Note (PDF 3665 kb)
Supplementary Table 1
Additional genomic regions showing suggestive evidence for association with IgAD. (XLS 32 kb)
Supplementary Table 2
Genetic association of previously validated non-HLA autoimmunity loci in IgA. (XLS 79 kb)
Supplementary Table 4
Genetic association of loci associated with non-autoimmune phenotypes in IgAD. (XLS 26 kb)
Rights and permissions
About this article
Cite this article
Ferreira, R., Pan-Hammarström, Q., Graham, R. et al. Association of IFIH1 and other autoimmunity risk alleles with selective IgA deficiency. Nat Genet 42, 777–780 (2010). https://doi.org/10.1038/ng.644
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.644
This article is cited by
-
Autoimmunity in Primary Immunodeficiencies (PID)
Clinical Reviews in Allergy & Immunology (2022)
-
Pediatric Celiac Disease and Selective IgA Deficiency: Unexpected Sequence of Events
Journal of Clinical Immunology (2022)
-
The aberrant expression of CD45 isoforms and levels of sex hormones in systemic lupus erythematosus
Clinical Rheumatology (2022)
-
Regulation of RIG-I-like receptor-mediated signaling: interaction between host and viral factors
Cellular & Molecular Immunology (2021)
-
New susceptible locus, rs9428555, is associated with pediatric-onset immunoglobulin A nephropathy and immunoglobulin A vasculitis in Koreans
Genes & Genomics (2021)