Abstract
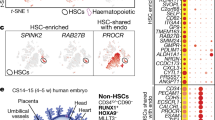
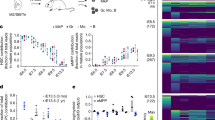
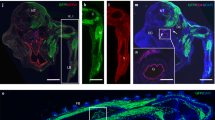
Haematopoietic and vascular cells are thought to arise from a common progenitor called the haemangioblast. Support for this concept has been provided by embryonic stem (ES) cell differentiation studies that identified the blast colony-forming cell (BL-CFC), a progenitor with both haematopoietic and vascular potential1,2. Using conditions that support the growth of BL-CFCs, we identify comparable progenitors that can form blast cell colonies (displaying haematopoietic and vascular potential) in gastrulating mouse embryos. Cell mixing and limiting dilution analyses provide evidence that these colonies are clonal, indicating that they develop from a progenitor with haemangioblast potential. Embryo-derived haemangioblasts are first detected at the mid-streak stage of gastrulation and peak in number during the neural plate stage. Analysis of embryos carrying complementary DNA of the green fluorescent protein targeted to the brachyury locus demonstrates that the haemangioblast is a subpopulation of mesoderm that co-expresses brachyury (also known as T) and Flk-1 (also known as Kdr). Detailed mapping studies reveal that haemangioblasts are found at highest frequency in the posterior region of the primitive streak, indicating that initial stages of haematopoietic and vascular commitment occur before blood island development in the yolk sac.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Choi, K., Kennedy, M., Kazarov, A., Papadimitriou, J. C. & Keller, G. A common precursor for hematopoietic and endothelial cells. Development 125, 725–732 (1998)
Kennedy, M. et al. A common precursor for primitive and definitive hematopoiesis. Nature 386, 488–493 (1997)
Millauer, B. et al. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 72, 835–846 (1993)
Yamaguchi, T. P., Dumont, D. J., Conlon, R. A., Breitman, M. L. & Rossant, J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development 118, 489–498 (1993)
Kabrun, N. et al. Flk-1 expression defines a population of early embryonic hematopoietic precursors. Development 124, 2039–2048 (1997)
Kallianpur, A. R., Jordan, J. E. & Brandt, S. J. The SCL/TAL-1 gene is expressed in progenitors of both the hematopoietic and vascular systems during embryogenesis. Blood 83, 1200–1208 (1994)
Silver, L. & Palis, J. Initiation of murine embryonic erythropoiesis: a spatial analysis. Blood 89, 1154–1164 (1997)
Breier, G. et al. Molecular cloning and expression of murine vascular endothelial-cadherin in early stage development of cardiovascular system. Blood 87, 630–641 (1996)
Orkin, S. H. GATA-binding transcription factors in hematopoietic cells. Blood 80, 575–581 (1992)
Robertson, S. M., Kennedy, M., Shannon, J. M. & Keller, G. A transitional stage in the commitment of mesoderm to hematopoiesis requiring the transcription factor SCL/tal-1. Development 127, 2447–2459 (2000)
Wilkinson, D. G., Bhatt, S. & Herrmann, B. G. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature 343, 657–659 (1990)
Lyons, I. et al. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2–5. Genes Dev. 9, 1654–1666 (1995)
Sitzmann, J., Noben-Trauth, K. & Klempnauer, K.-H. Expression of mouse c-myb during embryonic development. Oncogene 11, 2273–2279 (1995)
Zhang, J. C. et al. Analysis of SM22alpha-deficient mice reveals unanticipated insights into smooth muscle cell differentiation and function. Mol. Cell. Biol. 21, 1336–1344 (2001)
Duband, J. L., Gimona, M., Scatena, M., Sartore, S. & Small, J. V. Calponin and SM 22 as differentiation markers of smooth muscle: spatiotemporal distribution during avian embryonic development. Differentiation 55, 1–11 (1993)
Ema, M. et al. Combinatorial effects of Flk1 and Tal1 on vascular and hematopoietic development in the mouse. Genes Dev. 17, 380–393 (2003)
Fehling, H. J. et al. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development 130, 4217–4227 (2003)
Herrmann, B. G. Expression pattern of the Brachyury gene in whole-mount TWis/TWis mutant embryos. Development 113, 913–917 (1991)
Biben, C. et al. Murine cerberus homologue mCer-1: a candidate anterior patterning molecule. Dev. Biol. 194, 135–151 (1998)
Belo, J. A. et al. Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech. Dev. 68, 45–57 (1997)
Saga, Y. et al. MesP1: a novel basic helix-loop-helix protein expressed in the nascent mesodermal cells during mouse gastrulation. Development 122, 2769–2778 (1996)
Shalaby, F. et al. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell 89, 981–990 (1997)
Kinder, S. J. et al. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development 126, 4691–4701 (1999)
Cumano, A., Dieterlen-Lièvre, F. & Godin, I. Lymphoid potential, probed before circulation in mouse is restricted to caudal intraembryonic splanchnopleura. Cell 86, 907–916 (1996)
Cumano, A., Ferraz, J., Klaine, M., Di Santo, J. & Godin, I. Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity 15, 477–485 (2001)
Palis, J., Roberston, S., Kennedy, M., Wall, C. & Keller, G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 126, 5073–5084 (1999)
Downs, K. M. & Davies, T. Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development 118, 1255–1266 (1993)
Brady, G. & Iscove, N. N. Construction of cDNA libraries from single cells. Methods Enzymol. 225, 611–623 (1993)
Acknowledgements
We thank P. Gadue, S. Irion and S. Kattman for critical reading of this manuscript. We also thank the Mount Sinai Flow Cytometry Shared Research Facility for sorting assistance. This work was supported by NIH grants (G.K. and J.P.). H.J.F. is supported by grants from a Sonderforschungsbereich (SFB) and the IZKF Ulm.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Huber, T., Kouskoff, V., Joerg Fehling, H. et al. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature 432, 625–630 (2004). https://doi.org/10.1038/nature03122
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03122
This article is cited by
-
Generating hematopoietic cells from human pluripotent stem cells: approaches, progress and challenges
Cell Regeneration (2023)
-
A genome-wide relay of signalling-responsive enhancers drives hematopoietic specification
Nature Communications (2023)
-
In vivo clonal tracking reveals evidence of haemangioblast and haematomesoblast contribution to yolk sac haematopoiesis
Nature Communications (2023)
-
Gastruloids as in vitro models of embryonic blood development with spatial and temporal resolution
Scientific Reports (2022)
-
Why is endothelial resilience key to maintain cardiac health?
Basic Research in Cardiology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.