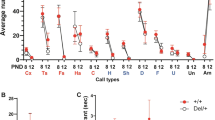
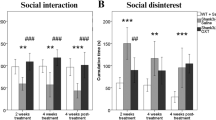
Abstract
A critical step toward understanding autism spectrum disorder (ASD) is to identify both genetic and environmental risk factors. A number of rare copy number variants (CNVs) have emerged as robust genetic risk factors for ASD, but not all CNV carriers exhibit ASD and the severity of ASD symptoms varies among CNV carriers. Although evidence exists that various environmental factors modulate symptomatic severity, the precise mechanisms by which these factors determine the ultimate severity of ASD are still poorly understood. Here, using a mouse heterozygous for Tbx1 (a gene encoded in 22q11.2 CNV), we demonstrate that a genetically triggered neonatal phenotype in vocalization generates a negative environmental loop in pup–mother social communication. Wild-type pups used individually diverse sequences of simple and complicated call types, but heterozygous pups used individually invariable call sequences with less complicated call types. When played back, representative wild-type call sequences elicited maternal approach, but heterozygous call sequences were ineffective. When the representative wild-type call sequences were randomized, they were ineffective in eliciting vigorous maternal approach behavior. These data demonstrate that an ASD risk gene alters the neonatal call sequence of its carriers and this pup phenotype in turn diminishes maternal care through atypical social communication. Thus, an ASD risk gene induces, through atypical neonatal call sequences, less than optimal maternal care as a negative neonatal environmental factor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Green J, Charman T, Pickles A, Wan MW, Elsabbagh M, Slonims V et al. Parent-mediated intervention versus no intervention for infants at high risk of autism: a parallel, single-blind, randomised trial. Lancet Psychiatry 2015; 2: 133–140.
Rogers SJ, Vismara L, Wagner AL, McCormick C, Young G, Ozonoff S . Autism treatment in the first year of life: a pilot study of infant start, a parent-implemented intervention for symptomatic infants. J Autism Dev Disord 2014; 44: 2981–2995.
Wetherby AM, Guthrie W, Woods J, Schatschneider C, Holland RD, Morgan L et al. Parent-implemented social intervention for toddlers with autism: an RCT. Pediatrics 2014; 134: 1084–1093.
Jones W, Klin A . Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature 2013; 504: 427–431.
Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T et al. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry 2010; 49: 256–266.
Bryson SE, Zwaigenbaum L, Brian J, Roberts W, Szatmari P, Rombough V et al. A prospective case series of high-risk infants who developed autism. J Autism Dev Disord 2007; 37: 12–24.
Soltis J . The signal functions of early infant crying. Behav Brain Sci 2004; 27: 443–458.
Zeifman DM . An ethological analysis of human infant crying: answering Tinbergen's four questions. Dev Psychobiol 2001; 39: 265–285.
Arriaga G, Jarvis ED . Mouse vocal communication system: are ultrasounds learned or innate? Brain Lang 2013; 124: 96–116.
Fischer J, Hammerschmidt K . Ultrasonic vocalizations in mouse models for speech and socio-cognitive disorders: insights into the evolution of vocal communication. Genes Brain Behav 2011; 10: 17–27.
Portfors CV, Perkel DJ . The role of ultrasonic vocalizations in mouse communication. Curr Opin Neurobiol 2014; 28: 115–120.
Brors D, Hansen S, Mlynski R, Volkenstein S, Aletsee C, Sendtner M et al. Spiral ganglion outgrowth and hearing development in p75-deficient mice. Audiol Neurootol 2008; 13: 388–395.
Hammerschmidt K, Reisinger E, Westekemper K, Ehrenreich L, Strenzke N, Fischer J . Mice do not require auditory input for the normal development of their ultrasonic vocalizations. BMC Neurosci 2012; 13: 40.
Scheiner E, Fischer J Emotion Expression: The Evolutionary Heritage in the Human Voice. In: Interdisciplinary Anthropology. Continuing Evolution of Man. Wolfgang Welsch, Wolf J. Singer, André Wunder (eds.). Springer-Verlag Berlin Heidelberg 2011, pp. 105–129..
Volkenstein S, Brors D, Hansen S, Berend A, Mlynski R, Aletsee C et al. Auditory development in progressive motor neuronopathy mouse mutants. Neurosci Lett 2009; 465: 45–49.
Esposito G, Venuti P . How is crying perceived in children with autistic spectrum disorder. Res Autism Spectr Disord 2008; 2: 371–384.
Esposito G, Venuti P . Comparative analysis of crying in children with autism, developmental delays, and typical development. Focus Autism Other Dev Disabil 2009; 24: 240–247.
Ozonoff S, Young GS, Belding A, Hill M, Hill A, Hutman T et al. The broader autism phenotype in infancy: when does it emerge? J Am Acad Child Adolesc Psychiatry 2014; 53: 398–407.
Okabe S, Nagasawa M, Kihara T, Kato M, Harada T, Koshida N et al. Pup odor and ultrasonic vocalizations synergistically stimulate maternal attention in mice. Behav Neurosci 2013; 127: 432–438.
Hiroi N, Takahashi T, Hishimoto A, Izumi T, Boku S, Hiramoto T . Copy number variation at 22q11.2: from rare variants to common mechanisms of developmental neuropsychiatric disorders. Mol Psychiatry 2013; 18: 1153–1165.
Schneider M, Debbane M, Bassett AS, Chow EW, Fung WL, van den Bree MB et al. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am J Psychiatry 2014; 171: 627–639.
Gong W, Gottlieb S, Collins J, Blescia A, Dietz H, Goldmuntz E et al. Mutation analysis of TBX1 in non-deleted patients with features of DGS/VCFS or isolated cardiovascular defects. J Med Genet 2001; 38: E45.
Ogata T, Niihori T, Tanaka N, Kawai M, Nagashima T, Funayama R et al. TBX1 mutation identified by exome sequencing in a Japanese family with 22q11.2 deletion syndrome-like craniofacial features and hypocalcemia. PLoS One 2014; 9 e91598.
Paylor R, Glaser B, Mupo A, Ataliotis P, Spencer C, Sobotka A et al. Tbx1 haploinsufficiency is linked to behavioral disorders in mice and humans: implications for 22q11 deletion syndrome. Proc Natl Acad Sci USA 2006; 103: 7729–7734.
Hiramoto T, Kang G, Suzuki G, Satoh Y, Kucherlapati R, Watanabe Y et al. Tbx1: identification of a 22q11.2 gene as a risk factor for autism spectrum disorder in a mouse model. Hum Mol Genet 2011; 20: 4775–4785.
Lai JK, Sobala-Drozdowski M, Zhou L, Doering LC, Faure PA, Foster JA . Temporal and spectral differences in the ultrasonic vocalizations of fragile X knock out mice during postnatal development. Behav Brain Res 2014; 259: 119–130.
Kihara T, Harada T, Kato M, Nakano K, Murakami O, Kikusui T et al. Reproduction of mouse-pup ultrasonic vocalzations by nanocrystalline silicon thermoacoustic emitter. Appl Phys Lett 2006; 88: 1–3.
Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ . Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 2013; 106-107: 1–16.
Scattoni ML, Gandhy SU, Ricceri L, Crawley JN . Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One 2008; 3: e3067.
Liao J, Kochilas L, Nowotschin S, Arnold JS, Aggarwal VS, Epstein JA et al. Full spectrum of malformations in velo-cardio-facial syndrome/DiGeorge syndrome mouse models by altering Tbx1 dosage. Hum Mol Genet 2004; 13: 1577–1585.
Uematsu A, Kikusui T, Kihara T, Harada T, Kato M, Nakano K et al. Maternal approaches to pup ultrasonic vocalizations produced by a nanocrystalline silicon thermo-acoustic emitter. Brain Res 2007; 1163: 91–99.
Young DM, Schenk AK, Yang SB, Jan YN, Jan LY . Altered ultrasonic vocalizations in a tuberous sclerosis mouse model of autism. Proc Natl Acad Sci USA 2010; 107: 11074–11079.
Ey E, Torquet N, Le Sourd AM, Leblond CS, Boeckers TM, Faure P et al. The autism ProSAP1/Shank2 mouse model displays quantitative and structural abnormalities in ultrasonic vocalisations. Behav Brain Res 2013; 256: 677–689.
Yang M, Mahrt EJ, Lewis F, Foley G, Portmann T, Dolmetsch RE et al. 16p11.2 deletion syndrome mice display sensory and ultrasonic vocalization deficits during social interactions. Autism Res 2015; 8: 507–521.
Burkett ZD, Day NF, Penagarikano O, Geschwind DH, White SA . VoICE: a semi-automated pipeline for standardizing vocal analysis across models. Sci Rep 2015; 5 10237.
Brigman JL, Graybeal C, Holmes A . Predictably irrational: assaying cognitive inflexibility in mouse models of schizophrenia. Front Neurosci 2010; 4: 19–28.
Sewell GD . Ultrasonic communication in rodents. Nature 1970; 227 410.
Ehret G, Haack B . Categorical perception of mouse pup ultrasound by lactating females. Naturwissenschaften 1981; 68: 208–209.
Ehret G . Categorical perception of mouse-pup ultrasounds in the temporal domain. Anim Behav 1992; 43: 409–416.
Ehret G, Haack B . Ultrasonic recognition in house mice: key-stimulus configuration and recognition mechanism. J Comp Physiol 1982; 148: 245–251.
Hammerschmidt K, Radyushkin K, Ehrenreich H, Fischer J . Female mice respond to male ultrasonic 'songs' with approach behaviour. Biol Lett 2009; 5: 589–592.
EHRET G . Infant rodent ultrasounds—a gate to the understanding of sound communication. Behav Genet 2005; 35: 19–29.
Blumberg MS, Sokoloff G . Do infant rats cry? Psychol Rev 2001; 108: 83–95.
Portfors CV . Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci 2007; 46: 28–34.
Schmeisser MJ, Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 2012; 486: 256–260.
Roy S, Watkins N, Heck D . Comprehensive analysis of ultrasonic vocalizations in a mouse model of fragile X syndrome reveals limited, call type specific deficits. PLoS One 2012; 7 e44816.
Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K et al. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell 2009; 137: 1235–1246.
Spruijt NE, Rana MS, Christoffels VM, Mink van der Molen AB . Exploring a neurogenic basis of velopharyngeal dysfunction in Tbx1 mutant mice: no difference in volumes of the nucleus ambiguus. Int J Pediatr Otorhinolaryngol 2013; 77: 1002–1007.
Wermke K, Mende W, Manfredi C, Bruscaglioni P . Developmental aspects of infant's cry melody and formants. Med Eng Phys 2002; 24: 501–514.
Fountain C, Winter AS, Bearman PS . Six developmental trajectories characterize children with autism. Pediatrics 2012; 129: e1112–e1120.
Gotham K, Pickles A, Lord C . Trajectories of autism severity in children using standardized ADOS scores. Pediatrics 2012; 130: e1278–e1284.
Szatmari P, Georgiades S, Duku E, Bennett TA, Bryson S, Fombonne E et al. Developmental trajectories of symptom severity and adaptive functioning in an inception cohort of preschool children with autism spectrum disorder. JAMA Psychiatry 2015; 72: 276–283.
Acknowledgements
We thank Bernice Morrow for providing us with the original line of Tbx1 heterozygous breeders. This work was supported by the NIH (HD053114 and MH099660), a NARSAD Independent Investigator Award to NH and a Maltz Foundation award to NH, a Grant-in-Aid for Scientific Research on Innovative Areas (No. 4501) from the Japan Society for the Promotion of Science, in Japan to TK; MB and JLP were supported by the NIH award (DC007690).
Author contributions
TT, AN, SA and NH contributed to the overall design and execution of experiments and analyses. TT, SO, PÓB, AN, KY, MVB, JLP, AG, TK and NH wrote the manuscript. GK and AN recorded pup vocalization and annotated call types. TT, TI and AN constructed all data files that were used for analyses. KY applied PLS-DA analysis to the proportion of vocal call types. PÓB and AG determined the sequence structure of vocal calls using sPLS-DA and entropy analyses. MVB and JLP analyzed call sequences using Markov chains. SO, AM and TK conducted the maternal approach experiment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Takahashi, T., Okabe, S., Broin, P. et al. Structure and function of neonatal social communication in a genetic mouse model of autism. Mol Psychiatry 21, 1208–1214 (2016). https://doi.org/10.1038/mp.2015.190
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.190
This article is cited by
-
Tbx1, a gene encoded in 22q11.2 copy number variant, is a link between alterations in fimbria myelination and cognitive speed in mice
Molecular Psychiatry (2022)
-
Gestational immune activation disrupts hypothalamic neurocircuits of maternal care behavior
Molecular Psychiatry (2022)
-
Computational identification of variables in neonatal vocalizations predictive for postpubertal social behaviors in a mouse model of 16p11.2 deletion
Molecular Psychiatry (2021)
-
Sex-dependent neurobiological features of prenatal immune activation via TLR7
Molecular Psychiatry (2020)
-
Specific profile of ultrasonic communication in a mouse model of neurodevelopmental disorders
Scientific Reports (2019)