Abstract
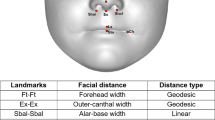
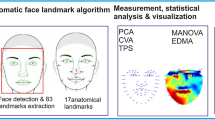
The heterogeneity of autism spectrum disorders (ASDs) confounds attempts to identify causes and pathogenesis. Identifiable endophenotypes and reliable biomarkers within ASDs would help to focus molecular research and uncover genetic causes and developmental mechanisms. We used dense surface-modelling techniques to compare the facial morphology of 72 boys with ASD and 128 first-degree relatives to that of 254 unrelated controls. Pattern-matching algorithms were able to discriminate between the faces of ASD boys and those of matched controls (AUC=0.82) and also discriminate between the faces of unaffected mothers of ASD children and matched female controls (AUC=0.76). We detected significant facial asymmetry in boys with ASD (P<0.01), notably depth-wise in the supra- and periorbital regions anterior to the frontal pole of the right hemisphere of the brain. Unaffected mothers of children with ASD display similar significant facial asymmetry, more exaggerated than that in matched controls (P<0.03) and, in particular, show vertical asymmetry of the periorbital region. Unaffected fathers of children with ASD did not show facial asymmetry to a significant degree compared to controls. Two thirds of unaffected male siblings tested were classified unseen as more facially similar to unrelated boys with ASD than to unrelated controls. These unaffected male siblings and two small groups of girls with ASD and female siblings, all show overall directional asymmetry, but without achieving statistical significance in two-tailed t-tests of individual asymmetry of ASD family and matched control groups. We conclude that previously identified right dominant asymmetry of the frontal poles of boys with ASD could explain their facial asymmetry through the direct effect of brain growth. The atypical facial asymmetry of unaffected mothers of children with ASD requires further brain studies before the same explanation can be proposed. An alternative explanation, not mutually exclusive, is a simultaneous and parallel action on face and brain growth by genetic factors. Both possibilities suggest the need for coordinated face and brain studies on ASD probands and their first-degree relatives, especially on unaffected mothers, given that their unusual facial asymmetry suggests an ASD susceptibility arising from maternal genes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Fombonne E . Epidemiological surveys of autism and other pervasive developmental disorders: an update. J Autism Dev Disord 2003; 33: 365–382.
Zoghbi HY . Postnatal neurodevelopmental disorders: meeting at the synapse? Science 2003; 302: 826–830.
DeMyer WE, Zeman W, Palmer CG . The face predicts the brain: diagnostic significance of median facial anomalies for holoprosencephaly (arhinencephaly). Pediatrics 1964; 34: 256–263.
Bookstein FL . Shape and information in medical images: a decade of the morphometric analysis. Comp Vis Im Understanding 1997; 66: 97–118.
Hammond P, Hutton TJ, Allanson JE, Campbell LE, Hennekam RC, Holden S et al. 3D analysis of facial morphology. Am J Med Genet A 2004; 126: 339–348.
Hennessy RJ, McLearie S, Kinsella A, Waddington JL . Facial surface analysis by 3D laser scanning and geometric morphometrics in relation to sexual dimorphism in cerebral-craniofacial morphogenesis and cognitive function. J Anat 2005; 207: 283–295.
Hutton TJ, Buxton BF, Hammond P, Potts HWW . Estimating average growth trajectories in shape-space using kernel smoothing. IEEE Trans Med Imaging 2003; 22: 747–753.
Hammond P, Hutton TJ, Allanson JE, Buxton B, Campbell L, Clayton-Smith J et al. Discriminating power of localized three-dimensional facial morphology. Am J Hum Genet 2005; 77: 999–1010.
Tassabehji M, Hammond P, Karmiloff-Smith A, Metcalfe K, Thompson P, Durkin M et al. GTF2IRD1 in craniofacial development of humans and mice. Science 2005; 310: 1184–1187.
Bhuiyan Z, Klein M, Hammond P, Mannens MMAM, van Berckelaer-Onnes I, Hennekam RCM . Phenotype–genotype correlations in Cornelia de Lange Syndrome: the Dutch experience. J Med Genet 2006; 43: 568–575.
Cox-Brinkman J, Vedder A, Hollak C, Richfield L, Mehta A, Orteu A et al. Three-dimensional face shape in Fabry disease. E J Hum Genet 2007; 15: 535–542.
Hammond P . The use of 3D face shape modelling in dysmorphology. Arch Dis Child 2007; 92: 1120–1126.
Lord C, Rutter M, Le Couteur A . Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Aut Dev Dis 1994; 24: 659–685.
Lord C, Risi S, Lambrecht L, Cook H, Leventhal BL, Pickles A, et al. Autism Diagnostic Observation Schedule-General (ADOS-G). J Aut Dev Dis 2000; 30: 205–223.
Gwilliam JR, Cunningham SJ, Hutton TJ . Reproducibility of soft tissue landmarks on three-dimensional facial scans. Eur J Ortho 2006; 28: 408–415.
Klingenberg CP, Barluenga M, Meyer A . Shape analysis of symmetric structures: quantifying variation among individuals and asymmetry. Evolution 2002; 56: 1909–1920.
Hennessy RJ, McLearie S, Kinsella A, Waddington JL . Facial shape and asymmetry by three dimensional laser surface scanning covary with cognition in a sexually dimorphic way. J Neuropsych Clin Neurosci 2006; 18: 73–80.
Baron-Cohen S, Ring H, Chitnis X, Wheelwright S, Gregory L, Williams S et al. fMRI of parents with Asperger Syndrome: a pilot study. Brain Cogn 2006; 61: 122–130.
Shackelford TK, Larsen RJ . Facial asymmetry as an indicator of psychological, emotional, and physiological distress. J Pers Soc Psychol 1997; 72: 456–466.
Toga AW, Thompson PM . Mapping brain asymmetry. Nat Rev Neuro 2003; 4: 37–48.
Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 2001; 57: 245–254.
Piven J, Arndt S, Bailey J, Havercamp S, Andreasen NC, Palmer P . An MRI study of brain size in autism. Am J Psych 1995; 152: 1145–1149.
Redcay E, Courchesne E . When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol Psych 2005; 59: 1–9.
Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ et al. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol 2004; 55: 530–540.
Carper RA, Courchesne E . Localized enlargement of the frontal cortex in early autism. Biol Psych 2005; 57: 126–133.
Herbert MR, Ziegler DA, Deutsch CK, O'Brien LM, Kennedy DN, Filipek PA et al. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain 2005; 128: 213–226.
Acknowledgements
This project was supported by operative funding from NewLife (PH), Fogarty/NIH (R21TW06761-01; PH); National Alliance for Autism Research-Autism Speaks (PH, MESL); the Canadian Institutes for Health Research (IHRT 43820, JJAH; no. RT-64217, MESL) and Canada Foundation for Innovation (no. 7939, JJAH). MESL is a Career Scholar funded by the Michael Smith Foundation for Health Research. We thank the families who volunteered for face scanning and the assistance of MJ Hildebrand and L Kasmara. Professors Andrew Wilkie, David Skuse and Annette Karmiloff-Smith provided helpful comments on early drafts of the manuscript. Dr Henry Potts and Dr Anthony Kinsella provided valuable comments on statistical analyses. We also thank the anonymous referees whose constructive criticism has resulted in significant improvements to the content, clarity and organization of the paper.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Rights and permissions
About this article
Cite this article
Hammond, P., Forster-Gibson, C., Chudley, A. et al. Face–brain asymmetry in autism spectrum disorders. Mol Psychiatry 13, 614–623 (2008). https://doi.org/10.1038/mp.2008.18
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2008.18
Keywords
This article is cited by
-
Facial asymmetry in dogs with fear and aggressive behaviors towards humans
Scientific Reports (2022)
-
Parents’ perspectives on the use of children’s facial images for research and diagnosis: a survey
Journal of Community Genetics (2022)
-
Brief Report: Facial Asymmetry and Autistic-Like Traits in the General Population
Journal of Autism and Developmental Disorders (2021)
-
A broad autism phenotype expressed in facial morphology
Translational Psychiatry (2020)
-
Dipyridamole-loaded 3D-printed bioceramic scaffolds stimulate pediatric bone regeneration in vivo without disruption of craniofacial growth through facial maturity
Scientific Reports (2019)