Abstract
The aim of this study was to investigate the clinical value of the apex beat and two ECG voltage criteria in the detection of left ventricular hypertrophy (LVH) while considering two distances, from the heart to the inner chest wall and to the chest surface, measured by using multislice CT (MSCT). The study population consisted of 151 patients clinically judged as requiring MSCT angiography. The apex beat was palpated with patients in the supine. Sokolow–Lyon voltage and Cornell voltage to detect LVH were determined. The pattern of sustained or double apical impulse and Cornell voltage had higher specificity as an indicator of LVH than Sokolow–Lyon voltage. Furthermore, the distance to the inner chest wall was negatively correlated with left ventricular end-diastolic volume and mass. Contrarily, the distance to the chest surface was correlated with the body mass index. Multivariate analyses revealed that the pattern of sustained or double apical impulse showed a stronger association with the distance to the inner chest wall than to the chest surface, but Sokolow–Lyon voltage was associated with the distance to the chest surface. Among the screening tests for excluding patients with LVH, Cornell voltage or the apex beat would be better than Sokolow–Lyon voltage because these are less dependent on body size and have higher specificity.
Similar content being viewed by others
Introduction
Several studies have shown that increased left ventricular (LV) mass is associated with a significant increase in the cardiovascular mortality and morbidity.1 To detect LV hypertrophy (LVH), two primitive non-invasive methods have been recommended as screening tests: 12-lead ECG and palpation of the apex beat. LVH detected on an ECG is a common manifestation of preclinical cardiovascular disease.2, 3 However, previous reports indicated that the Sokolow–Lyon voltage criteria had low sensitivity in the detection of anatomical LVH, which is defined as increased LV mass, and that the sensitivity of the Cornell voltage criteria to detect LVH was superior to the Sokolow–Lyon criteria while maintaining good specificity.4
On the other hand, although physicians frequently palpate the apex beat to evaluate cardiac size, function and LVH, the clinical significance of this diagnostic maneuver remains unclear. In general, the pattern of sustained or double apical impulse has been considered a sensitive indicator of LVH.5, 6 However, patients with a palpable apex beat are limited in number in the supine position because of the effect of body size. Recently, we demonstrated that the presence of an apex beat in the supine position was not only associated with LV mass but also with the distance from the heart to the chest wall and that in patients with a palpable apex beat, only LV mass was an independent factor associated with the pattern of sustained or double apical impulse.7 However, our previous study has not revealed which distance factors from the heart to the chest wall (to the inner chest wall or to the chest surface) were more strongly associated with the apex beat.
Recent advances in multislice CT (MSCT) have improved the spatial and temporal resolution of this technique, thereby enabling assessment of not only coronary artery stenosis and plaques but also LV function, volume, mass and the exact distance from the heart to the chest wall.7, 8, 9, 10 Thus far, few studies have evaluated the clinical value of palpating the apex beat and the ECG voltage criteria in the detection of LVH while considering two distances from the heart to the inner chest wall and to the chest surface. The present study has been designed for this purpose.
Methods
Patients
The study population consisted of 208 consecutive patients (159 males, 49 females; mean age±s.d., 65±10 years), without contraindications to MSCT, such as severe renal dysfunction or iodine contrast allergy, who underwent MSCT angiography for coronary artery evaluation between May 2007 and August 2008. All patients underwent palpation of the apex beat and electrocardiography during the week before the day on which CT angiography was performed. Patients with atrial fibrillation (N=2), history of myocardial infarction (N=51) or complete left bundle branch block (N=4) were excluded from the study. Thus, the study population consisted of 151 patients. Of these patients, 14 had undergone coronary artery bypass surgery, 10 had undergone percutaneous coronary intervention, 3 had hypertrophic cardiomyopathy, 5 had dilated cardiomyopathy and 5 had aortic valve stenosis.
The following data were collected: age, sex, presence of risk factors (smoking and hypertension, as defined by the Joint National Committee VII; diabetes mellitus, as defined by the World Health Organization Study Group; or hypercholesterolemia, as defined by the Japan Atherosclerosis Society Guidelines), body mass index (BMI) and blood pressure before image acquisition. The BMI was calculated by dividing the body weight (kg) by the square of height (m), and a BMI of ≥25.0 was defined as obesity. Informed consent was obtained from all patients before the study.
Physical diagnostic maneuvers
Apex beat palpation was performed with patients in the supine position. The point furthest down and outwards on the chest wall where the finger was lifted by a cardiac impulse was considered the point of the apex beat. When an impulse was palpable, the apex beat was categorized into three patterns: ‘tapping’, when it was palpable as a single, brief outward impulse; ‘sustained’, when it was associated with an outward impulse lasting up to or longer than the second heart sound; or ‘double apical impulse’, when one impulse was felt in early systole and another in diastole.5 The patients were examined by a cardiologist with more than 10 years of experience who was blind to the clinical history of the patient, ECG and MSCT findings. To determine agreement concerning pattern of the apex beat, a subset of 50 patients was also examined by a second cardiologist blind to the results of the first investigator and to clinical history and ECG and MSCT findings. Concordance between investigators, measured as a weighted κ statistic for the classification of patterns of the apex beat into four categories (no apex beat, tapping pattern, sustained pattern and double apical impulse), was substantial (0.88; 95% confidence interval, 0.78–0.98) in the supine position.
Electrocardiography
All resting 12-lead ECGs were obtained using standard amplifications, filter settings and paper speed. ECGs were interpreted by an experienced reader (TO) who was blinded to the clinical information. QRS amplitudes were measured to the nearest 0.5 mm (0.05 mV). The widely used ECG criteria for the detection of LVH were measured: Sokolow–Lyon voltage (SV1+RV5 or V6≥3.5 mV)11 and sex-specific Cornell voltage (RaVL+SV3≥2.8 mV in men or ≥2.0 mV in women).12
MSCT image acquisition, reconstruction and analysis
The patients were scanned in the supine position during a single breath hold by using a 64-slice CT scanner (SOMATOM Sensation 64; Siemens Medical Solutions, Forchheim, Germany). Patients with a heart rate of >65 beats min−1 received 20–60 mg metoprolol orally 2 h before the MSCT scan (128/151; 85%). In addition, all patients received 0.6 mg nitroglycerin sublingually immediately before scanning.
For coronary CT angiography, 65–85 ml of contrast medium (Iopamiron 370; Bayer HealthCare, Berlin, Germany) was injected through a dual-head injector into the cubital vein at the rate of 3.5–4.5 ml s−1, depending on the body weight; thereafter, 30 ml of saline solution chaser was injected. CT examination was performed with a tube voltage of 120 kV, an effective tube current time product of 770 effective mAs, a collimation of 64 × 0.6 mm, a pitch of 0.2 and a gantry rotation time of 330 ms.
We measured the LV end-diastolic volume (EDV), end-systolic volume (ESV), ejection fraction and LV mass by using a commercially available software (Syngo Circulation; Siemens) and indexed these parameters to the body surface area; this software has been previously validated.8, 13 For LV volume analyses, serial axial images (slice thickness, 2.0 mm) were reconstructed from the raw data for every 10% (0–90%) of the R–R interval. A field of view of 200 × 200 mm2, 512 × 512 matrix and medium smooth convolution kernel (B25f) were applied. Anatomical LVH was diagnosed if the LV mass index measured by MSCT was >104 g m−2 in women or greater than 116 g m−2 in men.2, 7
We then measured the distances from the LV apex to the inner chest wall and to the chest surface according to the method described in a previous report.7 Briefly, the smallest line from the LV apex to the inner chest wall was identified visually from the axial images obtained in the early systolic phase. Then, the sagittal image, in which the plane passes through this line, was used to measure the distances from the LV apex to the inner chest wall and to the chest surface.
Statistical analyses
The results are presented as mean±s.d. The two groups were compared with an unpaired Student’s t-test or with Mann–Whitney U-test when the variance was heterogeneous. Statistical comparisons among three groups were performed by one-way analysis of variance and post hoc multiple comparisons by Scheffe's test. Categorical variables were compared by the χ2-test. The relation between variables was assessed using Pearson's correlation coefficient. Using LV mass measured by MSCT as a gold standard, sensitivities, specificities, and positive and negative predictive values of the pattern of sustained or double apical impulse and ECG voltage criteria as indicators of anatomic LVH were calculated according to standard methods. To assess the distance factors (from the LV apex to the inner chest wall or to the chest surface) associated with the patterns of sustained or double apical impulses, Sokolow–Lyon voltage or Cornell voltage after removing the effect of LV mass, multivariate logistic regression and regression analyses were performed. P-values of <0.05 were considered significant.
Results
Clinical and CT findings classified by the patterns of the apex beat and ECG voltage criteria
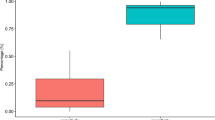
Table 1 shows the clinical characteristics and CT findings classified by the patterns of the apex beat. Of the 151 patients, 104 (69%) did not have a palpable apex beat. Among patients (N=47) with a palpable apex beat in the supine position, 17 patients (11%) had a tapping pattern and 30 (20%) had a sustained or double apical impulse. The incidence of male gender in patients without a palpable apex beat was significantly higher than that in patients with a palpable apex beat. Systolic blood pressure, LV EDV (index), ESV (index) and LV mass (index) in patients with either sustained pattern or double apical impulse were significantly greater than those in patients with a tapping pattern and those without a palpable apex beat. In addition, the distances to the inner chest wall (P<0.0001) and to the chest surface (P<0.05) in patients with either the sustained pattern or double apical impulse were significantly lesser than those in patients without a palpable apex beat.
Clinical and CT characteristics by the two ECG voltage criteria were shown in Table 2. The incidence of diabetes mellitus in patients with LVH defined according to the Sokolow–Lyon voltage criteria was significantly lower than that in patients determined as not having LVH by the same criteria. Systolic blood pressure, LV EDV (index) and LV mass (index) in patients with LVH defined according to the Sokolow–Lyon voltage criteria were significantly larger and the distance to the inner chest wall in these patients was significantly smaller than that in patients determined as not having LVH. In contrast, the incidence of obesity, systolic blood pressure, LV EDV (index), ESV (index) and LV mass (index) in patients with LVH defined according to the Cornell voltage criteria were significantly larger, and the distance to the inner chest wall in these patients was significantly smaller than that in patients determined as not having LVH by the same criteria.
Performance of the apex beat and ECG voltage criteria in the detection of LVH
Table 3 shows the sensitivity, specificity and predictive values of the pattern of sustained or double apical impulse and ECG voltage criteria, as indicators of anatomic LVH for the entire study population and in the obese and non-obese subgroups. For the entire study group (N=151), the pattern of sustained or double apical impulse in the supine position as an indicator of LVH had sensitivity of 56%, specificity of 91%, positive predictive value of 63% and negative predictive value of 88%. Among patients (N=47) with a palpable apex beat, the corresponding values were 90, 58, 63 and 88%, respectively. The Sokolow–Lyon voltage criteria had sensitivity of 53%, specificity of 79%, positive predictive value of 42% and negative predictive value of 85%. In contrast, the Cornell voltage criteria had sensitivity of 56%, specificity of 92%, positive predictive value of 68% and negative predictive value of 88%. Further, the sensitivity and negative predictive value of the Sokolow–Lyon voltage criteria in obese patients (BMI ≥25.0) were lower and the specificity and positive predictive value in these patients were higher than those in non-obese ones.
The combination of the patterns of sustained or double apical impulse or Sokolow–Lyon voltage criteria as indicators of LVH had a sensitivity of 74%, a specificity of 74%, a positive predictive value of 45% and a negative predictive value of 91%. Moreover, the combination of the patterns of sustained or double apical impulse or Cornell voltage criteria had a sensitivity of 76%, a specificity of 85%, a positive predictive value of 59% and a negative predictive value of 93%.
Correlation between the distance to the inner chest wall or to the chest surface and the size of left ventricle or body
As shown in Figure 1, the distance from the heart to the inner chest wall showed a weak negative correlation with the EDV index (R=0.389, P<0.0001) and LV mass index (R=0.282, P<0.0005). However, no relation was observed between the distance to the inner chest wall and BMI. In contrast, the distance to the chest surface was moderately correlated with BMI (R=0.602, P<0.0001); however, there was only weak association between the distance to the chest surface and EDV index (R=0.168, P<0.05), and there was no association between that distance and LV mass index (Figure 2).
Multivariate analyses were performed to assess the distance factor associated with the patterns of sustained or double apical impulses, Sokolow–Lyon voltage or Cornell voltage after removing the effect of LV mass. Multivariate analysis revealed that the distance to the inner chest wall remained associated with the pattern of sustained or double apical impulse (Table 4a). On the other hand, the distance to the chest surface was associated with the Sokolow–Lyon voltage. The distance to neither the inner chest wall nor the chest surface was associated with the Cornell voltage (Table 4b).
Discussion
To the best of our knowledge, this is the first study to investigate simultaneously the clinical value of the apex beat and the two major ECG voltage criteria applied to detect increased LV mass while taking the distance factors from the heart to the chest wall into consideration. This study has two major novel findings. First, the distance to the inner chest wall was negatively correlated with measurements of the heart size, such as EDV index and LV mass index. In contrast, the distance to the chest surface was correlated with the body size, such as BMI. Second, the pattern of sustained or double apical impulse showed a stronger association with the distance to the inner chest wall than to the chest surface; however, the Sokolow–Lyon voltage was associated with the distance to the chest surface. Neither the distance to the inner chest wall nor to the chest surface was associated with the Cornell voltage. That is, it is likely that the apex beat may be directly affected by the heart size, and that Sokolow–Lyon voltage may be more strongly affected by the body size. These findings may partly explain why the pattern of sustained or double apical impulse has higher sensitivity and specificity as an indicator of LVH than Sokolow–Lyon voltage.
Simple ECG criteria based on voltage had low sensitivity for the detection of anatomical LVH and previous studies have shown large discrepancies in the diagnostic value of these criteria. The variations may be attributed to the differences in the definition of anatomical LVH used in these studies or the effect of clinical characteristics such as sex, smoking and body size. Moreover, some studies reported that the measurements by MSCT overestimated LV volume, mass and left atrial volume compared with echocardiography or MRI.14, 15 In this study, it is possible that those subjects diagnosed as anatomical LVH may represent lesser degrees of LV mass, which may lead to a skew in the results. Nevertheless, the overall superiority of Cornell voltage over Sokolow–Lyon voltage found in this study is in agreement with the results of previous studies. Horton et al.16 reported that the distance from the heart to the chest surface influences the amplitude of the recorded precordial voltage. They calculated the distance from the chest surface to mid-LV by using echocardiography. However, they did not consider the distance from the heart to the inner chest wall. Sokolow–Lyon voltage is the sum of two precordial voltages, both dependent on the distance between the heart and the electrode (chest surface). In this study, the Sokolow–Lyon voltage showed a stronger association with the distance to the chest surface, which was correlated with body size measurements, such as BMI, than with the distance to the inner chest wall. Therefore, the sensitivity and negative predictive value of Sokolow–Lyon voltage in obese patients were lower than those in the non-obese ones. In contrast, the specificity and positive predictive value of Sokolow–Lyon voltage in obese patients were higher than those in the non-obese ones. Because Cornell voltage is the sum of the precordial voltage and a limb voltage, which is less dependent on chest wall thickness, it is not surprising that the overall superiority of Cornell voltage was found in the previous4 and our studies.
As shown in our previous7 and present studies, the apex beat was present in only 31% (47/151), and for the entire study group, including both patients with and without a palpable apex beat, the sensitivity and positive predictive value for the detection of LVH was low. Therefore, many clinicians may consider apex beat palpation useless. However, the pulsation of the heart is transmitted to the inner chest wall, and the examiner can feel the apex beat. The apex beat is more strongly associated with the distance to the inner chest wall, which showed a direct correlation with the heart size. This implies that patients without a palpable apex beat have a smaller LV mass and a greater distance to the inner chest wall. These factors would influence the specificity in the entire patient group. Our present findings regarding the simple screening tests for excluding patients with LVH has clinical implications, especially in patients with hypertension. The Losartan Intervention For Endpoint study revealed that low values of ECG LVH during antihypertensive therapy are associated with a decreased likelihood of cardiovascular morbidity and mortality, and this is independent of the treatment modality and decrease in blood pressure in patients with hypertension.2 In general, comprehensive factors, such as severe underlying hypertension, long duration of hypertension or poor control of blood pressure, would lead to concentric LVH, which may have a more important impact than the history of hypertension. Therefore, the identification or exception of patients with LVH is of paramount importance for bedside clinical examination.
This study has several limitations. First, the apex beat was not assessed in the left lateral decubitus position in this study, because the main purpose of this study is to investigate the relationship between the apex beat and the distance factors from the heart to the chest wall. Second, although the κ value for the pattern of the apex beat as determined by physical examination was substantial (κ=0.88) in this study, this result depends on the skills of the examiners. Moreover, the definitions of apex beat pattern were not quantitative and highly subjective. Objective measurements and training on the apex beat pattern using methods such as apex cardiography may be important. Although we understand the limited clinical value of apex beat patterns, we would agree that it has served its time as a test for the estimation of LV mass. Finally, β-blocker or nitroglycerin before the MSCT scan would affect LV volume and function. However, the use of these medications was not avoided because our study population consisted of patients who were clinically judged as requiring MSCT angiography for coronary artery evaluation, and not for the analyses of LV parameters.
Conclusions
Palpation of the apex beat and ECG findings should be used for the detection of LVH while taking into consideration the following findings. The apex beat was more strongly associated with the distance to the inner chest wall, which showed a direct correlation with the heart size. Furthermore, Sokolow–Lyon voltage showed a stronger association with the distance to the chest surface, which in turn was correlated with the body size. Neither the distance to the inner chest wall nor to the chest surface was associated with the Cornell voltage. In a real world setting, clinicians would accept the findings that the tapping pattern or absence of an apex beat excludes the presence of LVH with a high probability, and that the emphasis should be on body size when interpreting the Sokolow–Lyon voltage.
References
Levy D, Garrison R, Savage DD, Kannel WB, Castelli WP . Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990; 322: 1561–1566.
Okin PM, Devereux RB, Nieminen MS, Jern S, Oikarinen L, Viitasalo M, Toivonen L, Kjeldsen SE, Julius S, Dahlöf B . Relationship of the electrocardiographic strain pattern to left ventricular structure and function in hypertensive patients: the LIFE study. Losartan Intervention For End point. J Am Coll Cardiol 2001; 38: 514–520.
Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, Snapinn S, Harris KE, Aurup P, Edelman JM, Wedel H, Lindholm LH, Dahlöf B . Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA 2004; 292: 2343–2349.
Abergel E, Tase M, Menard J, Chatellier G . Influence of obesity on the diagnostic value of electrocardiographic criteria for detecting left ventricular hypertrophy. Am J Cardiol 1996; 77: 739–744.
Beilin L, Mounsey P . The left ventricular impulse in hypertensive heart disease. Br Heart J 1962; 24: 409–421.
Lindroos M, Kupari M, Heikkilä J, Tilvis R . Echocardiographic evidence of left ventricular hypertrophy in a general aged population. Am J Cardiol 1994; 74: 385–390.
Ehara S, Okuyama T, Shirai N, Oe H, Matsumura Y, Sugioka K, Itoh T, Otani K, Hozumi T, Yoshiyama M, Yoshikawa J . Comprehensive evaluation of the apex beat using 64-slice computed tomography: impact of left ventricular mass and distance to chest wall. J Cardiol 2010; 55: 256–265.
Okuyama T, Ehara S, Shirai N, Sugioka K, Ogawa K, Oe H, Kitamura H, Itoh T, Otani K, Matsuoka T, Inoue Y, Ueda M, Hozumi T, Yoshiyama M . Usefulness of three-dimensional automated quantification of left ventricular mass, volume, and function by 64-slice computed tomography. J Cardiol 2008; 52: 276–284.
Wu YW, Tadamura E, Kanao S, Yamamuro M, Okayama S, Ozasa N, Toma M, Kimura T, Kita T, Marui A, Komeda M, Togashi K . Left ventricular function analysis using 64-slice multidetector row computed tomography: comparison with left ventriculography and cardiovascular magnetic resonance. Cardiology 2008; 109: 135–142.
Wu YW, Tadamura E, Yamamuro M, Kanao S, Okayama S, Ozasa N, Toma M, Kimura T, Komeda M, Togashi K . Estimation of global and regional cardiac function using 64-slice computed tomography: a comparison study with echocardiography, gated-SPECT and cardiovascular magnetic resonance. Int J Cardiol 2008; 128: 69–76.
Sokolow M, Lyon TP . The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949; 37: 161–186.
Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P . Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation 1987; 75: 565–572.
Mühlenbruch G, Das M, Hohl C, Wildberger JE, Rinck D, Flohr TG, Koos R, Knackstedt C, Günther RW, Mahnken AH . Global left ventricular function in cardiac CT Evaluation of an automated 3D region-growing segmentation algorithm. Eur Radiol 2006; 16: 1117–1123.
Schlosser T, Mohrs OK, Magedanz A, Voigtländer T, Schmermund A, Barkhausen J . Assessment of left ventricular function and mass in patients undergoing computed tomography (CT) coronary angiography using 64-detector-row CT: comparison to magnetic resonance imaging. Acta Radiol 2007; 48: 30–35.
Takagi Y, Ehara S, Okuyama T, Shirai N, Yamashita H, Sugioka K, Kitamura H, Ujino K, Hozumi T, Yoshiyama M . Comparison of determinations of left atrial volume by the biplane area-lengthy and Simpson’s methods using 64-slice computed tomography. J Cardiol 2009; 53: 257–264.
Horton JD, Sherber HS, Lakatta EG . Distance correction for precordial electrocardiographic voltage in estimating left ventricular mass: an echocardiographic study. Circulation 1977; 55: 509–512.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Ehara, S., Shirai, N., Matsumoto, K. et al. The clinical value of apex beat and electrocardiography for the detection of left ventricular hypertrophy from the standpoint of the distance factors from the heart to the chest wall: a multislice CT study. Hypertens Res 34, 1004–1010 (2011). https://doi.org/10.1038/hr.2011.69
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2011.69