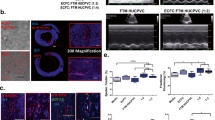
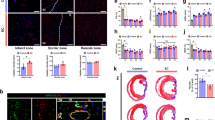
Abstract
Left ventricular remodeling is a major cause of progressive heart failure and death after myocardial infarction. Although neoangiogenesis within the infarcted tissue is an integral component of the remodeling process, the capillary network is unable to support the greater demands of the hypertrophied myocardium, resulting in progressive loss of viable tissue, infarct extension and fibrous replacement. Here we show that bone marrow from adult humans contains endothelial precursors with phenotypic and functional characteristics of embryonic hemangioblasts, and that these can be used to directly induce new blood vessel formation in the infarct-bed (vasculogenesis) and proliferation of preexisting vasculature (angiogenesis) after experimental myocardial infarction. The neoangiogenesis resulted in decreased apoptosis of hypertrophied myocytes in the peri-infarct region, long-term salvage and survival of viable myocardium, reduction in collagen deposition and sustained improvement in cardiac function. The use of cytokine-mobilized autologous human bone-marrow–derived angioblasts for revascularization of infarcted myocardium (alone or in conjunction with currently used therapies) has the potential to significantly reduce morbidity and mortality associated with left ventricular remodeling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Mahon, N.G. et al. Hospital mortality of acute myocardial infarction in the thrombolytic era. Heart 81, 478–482 (1999).
Pfeffer, J.M., Pfeffer, M.A., Fletcher, P.J. & Braunwald, E. Progressive ventricular remodeling in rat with myocardial infarction. Am. J. Physiol. 260, H1406–1414 (1991).
White, H.D. et al. Left ventricular end systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation 76, 44–51 (1987).
Colucci, W.S. Molecular and cellular mechanisms of myocardial failure. Am. J. Cardiol. 80, 15L–25L (1997).
Ravichandran, L.V. & Puvanakrishnan, R. In vivo labeling studies on the biosynthesis and degradation of collagen in experimental myocardial myocardial infarction. Biochem. Intl. 24, 405–414 (1991).
Agocha, A., Lee, H.W. & Eghali-Webb, M. Hypoxia regulates basal and induced DNA synthesis and collagen type I production in human cardiac fibroblasts: effects of TGF-β, thyroid hormone, angiotensis II and basic fibroblast growth factor. J. Mol. Cell. Cardiol. 29, 2233–2244H (1997).
Tomita, S. et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 100, 247–256 (1999).
Liechty, K.W. et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nature Med. 6, 1282–1286 (2000).
Pittenger, M.F. et al. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 (1999).
Makino, S. et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J. Clin. Invest. 103, 697–705 (1999).
Nelissen-Vrancken, H., Debets, J., Snoeckx, L., Daemen, M. & Smits, J. Time-related normalization of maximal coronary flow in isolated perfused hearts of rats with myocardial infarction. Circulation 93, 349–355 (1996).
Kalkman, E.A.J. et al. Determinants of coronary reserve in rats subjected to coronary artery ligation or aortic banding. Cardiovasc. Res. 32, 1088–1095 (1996).
Heymans, S. et al. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nature Med. 5, 1135–1142 (1999).
Hochman, J.S. & Choo, H. Limitation of myocardial infarct expansion by reperfusion independent of myocardial salvage. Circulation 75, 299–306 (1987).
White, H.D. et al. Long-term prognostic importance of patency of the infarct-related coronary artery after thrombolytic therapy for myocardial infarction. Circulation 89, 61–67 (1994).
Nidorf, S.M., Siu, S.C., Galambos, G., Weyman, A.E. & Picard, M.H. Benefit of late coronary reperfusion on ventricular morphology and function after myocardial infarction. J. Am. Coll. Cardiol. 20, 307–313 (1992).
Folkman, J. Therapeutic angiogenesis in ischemic limbs. Circulation 97, 1108–10 (1998).
Asahara, T. et al. Isolation of putative progenitor cells for endothelial angiogenesis. Science 275, 964–967 (1997).
Takahashi, T. et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nature Med. 5, 434–438 (1999).
Kalka, C. et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl. Acad. Sci. USA 97, 3422–3427 (2000).
Rafii, S. et al. Isolation and characterization of human bone marrow microvascular endothelial cells: hematopoietic progenitor cell adhesion. Blood 84, 10–19 (1994).
Shi, Q. et al. Evidence for circulating bone marrow-derived endothelial cells. Blood 92, 362–367 (1998).
Lin, Y., Weisdorf, D.J., Solovey, A. & Hebbel, R.P. Origins of circulating endothelial cells and endothelial outgrowth from blood. J. Clin. Invest. 105, 71–77 (2000).
Tavian, M. et al. Aorta-associated CD34 hematopoietic cells in the early human embryo. Blood 87, 67–72 (1996).
Jaffredo, T., Gautier, R., Eichmann, A. & Dieterlen-Lievre, F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development 125, 4575–4583 (1998).
Kennedy, M. et al. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature 386, 488–493 (1997).
Choi, K., Kennedy, M., Kazarov, A., Papadimitriou, N. & Keller, G. A common precursor for hematopoietic and endothelial cells. Development 125, 725–732 (1998).
Elefanty, A.G., Robb, L., Birner, R. & Begley, C.G. Hematopoietic-specific genes are not induced during in vitro differentiation of scl-null embryonic stem cells. Blood 90, 1435–1447 (1997).
Labastie, M.C., Cortes, F., Romeo, P.H., Dulac, C. & Peault, B. Molecular identity of hematopoietic precursor cells emerging in the human embryo. Blood 92, 3624–3635 (1998).
Tsai, F.Y. et al. An early hematopoietic defect in mice lacking the transcription factor GATA-2. Nature 371, 221–225 (1994).
Ogawa, M. et al. Expression of α4-integrin defines the earliest precursor of hematopoietic cell lineage diverged from endothelial cells. Blood 93, 1168–1177 (1999).
Peichev, M. et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 95, 952–958 (2000).
Asahara, T. et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 18, 3964–3972 (1999).
Olivetti, G., Capasso, J.M., Meggs, L.G., Sonnenblick, E.H. & Anversa, P. Cellular basis of chronic ventricular remodeling after myocardial infarction in rats. Circ. Res. 68, 856–869 (1991).
Braunwald, E. & Pfeffer, M.A. Ventricular enlargement and remodelling following acute myocardial infarction: mechanisms and management. Am. J. Cardiol. 68, 1–6D (1991).
Narula, J. et al. Apoptosis in myocytes in end-stage heart failure. N. Engl. J. Med. 335, 1182–1189 (1996).
Cheng, W. et al. Programmed myocyte cell death affects the viable myocardium after infarction in rats. Exp. Cell. Res. 226, 316–327 (1996).
Hart, P.H. et al. Activation of human monocytes by granulocyte-macrophage colony-stimulating factor: increased urokinase-type plasminogen activator activity. Blood 77, 841–848 (1991).
Stacey, K.J. et al. Regulation of urokinase-type plasminogen activator gene transcription by macrophage colony-stimulating factor. Mol. Cell. Biol. 15, 3430–3441 (1995).
Pei, X.H. et al. G-CSF increases secretion of urokinase-type plasminogen activator by human lung cancer cells. Clin. Exp. Metastasis 16, 551–558 (1998).
Dorfman, D.M., Wilson, D.B., Bruns, G.A. & Orkin, S.H. Human transcription factor GATA-2. Evidence for regulation of preproendothelin-1 gene expression in endothelial cells. J. Biol. Chem. 267, 1279–1285 (1992).
Ito, H. et al. Endothelin-1 is an autocrine/paracrine factor in the mechanism of angiotensin II-induced hypertrophy in cultured rat cardiomyocytes. J. Clin. Invest. 92, 398–403 (1993).
Kalkman, E.A.J., van Haren, P., Saxena, P.R. & Schoemaker, R.G. Regionally different vascular response to vasoactive substances in the remodeled infarcted rat heart; aberrant vasculature in the infarct scar. J. Mol. Cell. Cardiol. 29, 1487–1497 (1997).
McEwan, P.E., Gray, G.A., Sherry, L., Webb, D.J. & Kenyon, C.J. Differential effects of angiotensin II on cardiac cell proliferation and intramyocardial perivascular fibrosis in vivo. Circulation 98, 2765–2773 (1998).
Kawano, H. et al. Angiotensin II has multiple profibrotic effects in human cardiac fibroblasts. Circulation 101, 1130–1137 (2000).
Pfeffer, M.A. et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE investigators. N. Engl. J. Med. 327, 669–677 (1992).
The SOLVD investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N. Engl. J. Med. 325, 293–302 (1991).
Pitt, B. et al. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE). Lancet 349, 747–752 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kocher, A., Schuster, M., Szabolcs, M. et al. Neovascularization of ischemic myocardium by human bone-marrow–derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 7, 430–436 (2001). https://doi.org/10.1038/86498
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/86498
This article is cited by
-
Effect of once versus twice intracoronary injection of allogeneic-derived mesenchymal stromal cells after acute myocardial infarction: BOOSTER-TAHA7 randomized clinical trial
Stem Cell Research & Therapy (2023)
-
Transplantation of mesenchymal stem cells for prevention of acute myocardial infarction induced heart failure: study protocol of a phase III randomized clinical trial (Prevent-TAHA8)
Trials (2022)
-
Single vs. double intracoronary injection of mesenchymal stromal cell after acute myocardial infarction: the study protocol from a randomized clinical trial: BOOSTER-TAHA7 trial
Trials (2022)
-
Purification and characterization of human adipose-resident microvascular endothelial progenitor cells
Scientific Reports (2022)
-
Kardiale Zelltherapie – „lost in translation?“
Zeitschrift für Herz-,Thorax- und Gefäßchirurgie (2022)