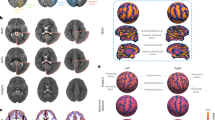
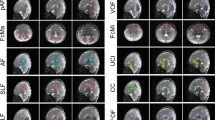
Abstract
The dynamic nature of growth and degenerative disease processes requires the design of sensitive strategies to detect, track and quantify structural change in the brain in its full spatial and temporal complexity1. Although volumes of brain substructures are known to change during development2, detailed maps of these dynamic growth processes have been unavailable. Here we report the creation of spatially complex, four-dimensional quantitative maps of growth patterns in the developing human brain, detected using a tensor mapping strategy with greater spatial detail and sensitivity than previously obtainable. By repeatedly scanning children (aged 3–15 years) across time spans of up to four years, a rostro-caudal wave of growth was detected at the corpus callosum, a fibre system that relays information between brain hemispheres. Peak growth rates, in fibres innervating association and language cortices, were attenuated after puberty, and contrasted sharply with a severe, spatially localized loss of subcortical grey matter. Conversely, at ages 3–6 years, the fastest growth rates occurred in frontal networks that regulate the planning of new actions. Local rates, profiles, and principal directions of growth were visualized in each individual child.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fox, N. C., Freeborough, P. A. & Rossor, M. N. Visualisation and quantification of rates of atrophy in Alzheimer's disease. Lancet 348, 94– 97 (1996).
Giedd, J. N. et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb. Cortex 6, 551– 560 (1996).
Yakovlev, P. I. & Lecours, A. R. in Regional Development of the Brain in Early Life (ed. Minkowski, A.) 3– 70 (Davis, Philadelphia, 1967).
Sowell, E. R., Thompson, P. M., Holmes, C. J., Jernigan, T. L. & Toga, A. W. In vivo evidence for post-adolescent brain maturation frontal and striatal regions. Nature Neurosci. 2, 859– 861 (1999).
Chugani, H. T., Phelps, M. E. & Mazziotta, J. C. Positron emission tomography study of human brain functional development. Ann. Neurol. 22, 487–497 (1987).
Grimshaw, G. M., Adelstein, A., Bryden, M. P. & MacKinnon, G. E. First-language acquisition in adolescence: evidence for a critical period for verbal language development. Brain Lang. 63, 237–255 (1998).
Thompson, P. M. et al. Cortical variability and asymmetry in normal aging and Alzheimer's disease. Cereb. Cortex 8, 492– 509 (1998).
Zijdenbos, A. P. & Dawant, B. M. Brain segmentation and white matter lesion detection in MR images. Crit. Rev. Biomed. Eng. 22, 401–465 ( 1994).
Woods, R. P., Cherry, S. R. & Mazziotta, J. C. Rapid automated algorithm for aligning and reslicing PET images. J. Comp. Assist. Tomogr. 16, 620–633 (1992).
Freeborough, P. A., Woods, R. P. & Fox, N. C. Accurate registration of serial 3D MR brain images and its application to visualizing change in neurodegenerative disorders. J. Comp. Assist. Tomogr. 20, 1012– 1022 (1996).
MacDonald, D., Avis, D. & Evans, A. C. in Proc. SPIE Conf. Visualization in Biomedical Computing (ed. Robb, R. A.) 2359, 160–169 (1994).
Thompson, P. M. & Toga, A. W. A surface-based technique for warping 3-dimensional images of the brain. IEEE Trans. Med. Imag. 15, 471–489 ( 1996).
Thompson, P. M. & Toga, A. W. Detection, visualization and animation of abnormal anatomic structure with a deformable probabilistic brain atlas based on random vector field transformations. Med. Image Anal. 1, 271–294 ( 1997).
Thompson, P. M. & Toga, A. W. in Brain Warping (ed. Toga, A. W.) 311–336 (Academic, San Diego, 1998).
Thompson, P. M., Schwartz, C., Lin, R. T., Khan, A. A. & Toga, A. W. 3D statistical analysis of sulcal variability in the human brain. J. Neurosci. 16, 4261– 4274 (1996).
Thompson, P. M. et al. Detection and mapping of abnormal brain structure with a probabilistic atlas of cortical surfaces. J. Comp. Assist. Tomogr. 21, 567–581 (1997).
Davatzikos, C. Spatial normalization of 3D brain images using deformable models. J. Comp. Assist. Tomogr. 20, 656–665 (1996).
Miller, M. I. & Grenander, U. Computational anatomy: an emerging discipline. Q. Appl. Math. 56, 617– 694 (1998).
Acknowledgements
We thank E. Sowell, M. Mega and J. Mazziotta for their advice and support. P.M.T. was supported by the Howard Hughes Medical Institute, the US Information Agency, and the US–UK Fulbright Commission. Additional research support was provided by a Human Brain Project grant to the International Consortium for Brain Mapping, funded jointly by NIMH and NIDA, by National Institutes of Health intramural funding (J.N.G.), and by the National Library of Medicine, National Science Foundation, and the NCRR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thompson, P., Giedd, J., Woods, R. et al. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature 404, 190–193 (2000). https://doi.org/10.1038/35004593
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35004593
This article is cited by
-
Are phonological skills as crucial for literacy acquisition in Japanese as in English as well as in accounting for developmental dyslexia in English and in Japanese?
Journal of Cultural Cognitive Science (2023)
-
Morphological changes in subregions of hippocampus and amygdala in major depressive disorder patients
Brain Imaging and Behavior (2020)
-
The splenium of the corpus callosum: embryology, anatomy, function and imaging with pathophysiological hypothesis
Neuroradiology (2020)
-
Effect of changing the analyzed image contrast on the accuracy of intracranial volume extraction using Brain Extraction Tool 2
Radiological Physics and Technology (2020)
-
Computing Univariate Neurodegenerative Biomarkers with Volumetric Optimal Transportation: A Pilot Study
Neuroinformatics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.