Hallux valgus (HV) is a lateral displacement of the great toe, resulting in deformity of the first metatarsophalangeal joint, with callus, bursa, or bunion formation over the bony prominence. Conservative management may include footwear modification and the use of insoles or toe spacers. Patients in whom conservative therapy fails to relieve symptoms may resort to surgery. However, consensus regarding the best management has yet to be established, and risk factors for developing HV are poorly understood (Ferrari et al., Reference Ferrari, Higgins and Prior2004; Nix et al., Reference Nix, Smith and Vicenzino2010). The etiology of HV is believed to be multifactorial. Studies about the risk factor for HV have reported consistent associations with female sex and older age (Coughlin, Reference Coughlin1997; Coughlin & Jones, Reference Coughlin and Jones2007; Krauss et al., Reference Krauss, Grau, Mauch, Maiwald and Horstmann2008; Pique-Vidal et al., Reference Pique-Vidal, Sole and Antich2007); other less replicated and potential risk factors of HV include high-heel or narrow toe box shoes (Coughlin, Reference Coughlin1997; Kato & Watanabe, Reference Kato and Watanabe1981), and flat feet (Coughlin, Reference Coughlin1995; Coughlin & Jones, Reference Coughlin and Jones2007; Pique-Vidal et al., Reference Pique-Vidal, Sole and Antich2007).
Previous studies about the etiology of HV, however, were mostly based on clinical cases, which are subject to selection bias unless the ascertainment of cases are not corrected properly (Hardy & Clapham, Reference Hardy and Clapham1951; Pique-Vidal et al., Reference Pique-Vidal, Sole and Antich2007), and the definition of HV relied on questionnaires, except a recent study that included radiologic examinations in a general population (Hannan et al., Reference Hannan, Menz, Jordan, Cupples, Cheng and Hsu2013).
The Healthy Twin study is a population-based twin-family cohort study not ascertained by health status. We conducted a study to estimate relative importance of genes and environments on HV among participants of the Healthy Twin study. We expect our findings will add insight to existing body of evidence regarding the etiology of HV.
Materials and Methods
Subjects
We recruited adult twins and their families who participated in the Healthy Twin study from May 2008 to April 2010. The Healthy Twin study was launched in 2005 and is an ongoing cohort study recruiting adult (≥30 years of age) same-sex twins and their first-degree relatives, based on a mail-out to a nation-wide twin register. Participants are invited to one of three general hospitals affiliated to medical schools, where they receive full medical examinations and complete detailed questionnaires about lifestyle and epidemiologic information. The Healthy Twin study has recruited more than 3,700 individuals as of January 2014, and the study is active in following up participants every 3 years. Details on the study design and protocols have been previously described (Sung et al., Reference Sung, Cho, Lee, Ha, Choi, Choi and Song2006). Physical examinations by a well-trained physiotherapist and radiographic measurements of feet were undertaken at the Samsung Medical Center. All participants provided written informed consent. The study protocol was approved by the Institutional Review Board at the Samsung Medical Center and Seoul National University School of Public Health.
Radiographic Assessment
Radiographs were taken in the anteroposterior standing positions (APS). Radiologic images were taken with the subjects standing on a cassette and bearing their weight. The X-ray source was centered on the midfoot and tilted 15 degrees toward the ankle joint. The HV angle (HVA) was measured in the anteroposterior weight-bearing view by a trained physiotherapist, and over 20 degrees of the HVA was defined as HV (Figure 1; Coughlin & Jones, Reference Coughlin and Jones2007; Pique-Vidal et al., Reference Pique-Vidal, Sole and Antich2007).

FIGURE 1 Measuring halux valgus angle (HVA) by radiography. HVA was measured as the angle between the line from the center of the metatarsal bone and the line connecting the midpoints of the longitudinal axis of the proximal phalanx.
Zygosity and Family Relationship Ascertainment
The sixteen short tandem repeat markers kit (PerkinElmer's AmpFlSTR®, Norwalk, CT) was used in order to ascertain the zygosity of twins and to confirm reported family relationships for 408 of the total Healthy Twin participants; for others, a zygosity questionnaire developed by the research team was used to determine the zygosity, which has more than 98% of predictive value for MZ twins (Song et al., Reference Song, Lee, Lee, Lee, Lee, Hong and Sung2010).
Counting Number of Pairs According to Relative Types
In order to estimate intraclass correlations for relative types, the number of family pairs were counted for all possible relationships, including twins or siblings; for example, as in Figure 2, if there are both parents (M & F), one twin pair (Tw1 & Tw2), and two singleton siblings (Br & Si), there are eight parent-offspring pairs (M-Br, M-Tw1, M-Tw2, M-Si, F-Br, F-Tw1, F-Tw2, F-Si) and five sibling pairs (Br-Si, Br-Tw1, Br-Tw2, Si-Tw1, Si-Tw2). Note that each cotwin will contribute to form a sibling pair with other singleton siblings; the number of family type pairs will therefore be different from the number of individuals.
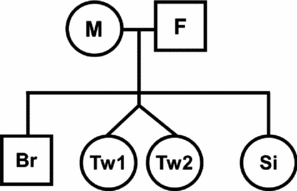
FIGURE 2 Method of counting family pairs in twin-family pedigree. Please read the method section for the detail.
Statistical Analyses
As an index of relative genetic contributions, heritability as the proportion of the total phenotypic variance explained by additive genetic factors was estimated. In order to estimate additive genetic contributions to the HV deformity, a variance-components method (VCM) implemented in the Sequential Oligogenic Linkage Analysis Routines software package (SOLAR, version 4.2.7; Southwest Foundation for Biomedical Research, San Antonio, TX, USA; http://solar.sfbrgenetics.org/;version 4.2.7) was used (Almasy & Blangero, Reference Almasy and Blangero1998). In this standard genetic analysis program, the degree of linear increase in the intraclass correlation explained by the increase in the kinship coefficient was regarded as the narrow sense heritability of the phenotype. Same first-degree relationships, that is, dizygotic twins (DZ) and siblings, were combined in ICC estimation. For comparisons between twin and singleton families, an age- and sex-standardized mean with the Korean population as a standard was calculated. For descriptive analyses, Student's t test, ANOVA or chi-square test were applied as appropriate.
Both HVA as quantitative traits and having HV or not as dichotomous traits were examined for their genetic and environmental contributions shared among family. Two cut-off values of diagnosing HV were used: over 20 degrees and 15 degrees. For dichotomous traits, the model assumed a continuous liability function based on the prevalence of the HV in this population. Intra-class correlations (ICC) between family relationships were calculated using a random effect model as a proportion of residual variance not explained by the particular family relationship over total phenotypic variance adjusted for age and sex, where each family was regarded as separate unit of random effects. For variance component methods analysis, both A (A: additive genetics; E: unshared unique environments) and ACE (C: shared environments within household, in addition to AE) models were compared, and the models which showed the smallest Akaike information criterion (AIC) value were selected as the best fitting model.
Result
A total of 1,265 individuals were included for this study, comprising 175 MZ twin pairs, 31 DZ pairs and 853 singleton family members of twins. We identified 959 parent-offspring pairs, and 574 sibling pairs in addition to twin pairs. The general characteristics of subjects and prevalence of HV are shown in Table 1; 38.7% (489 persons) were men, and the average age was 43.9 years for men and 44.1 for women. The prevalence of HV as HVA over 20 degrees was 16.4% (208 persons), while HV rate as HVA over 15 degrees was 44.0% (556 individuals), and the crude (Cr) and standardized (St) prevalence of HV in twins were 13.9% (Cr) and 16.2% (St), and for non-twins 14.3% (Cr) and 18.3% (St). Typical AP radiographs of a MZ twin pair with HV and their family members are shown in Supplementary Figure 1. The adjusted intraclass correlation coefficients for having HV or not by HVA over 20 degrees (HV-20, dichotomous), HVA over 15 degrees (HV-15, dichotomous), and HVA as it is (continuous values) were 0.51–0.53, 0.27–0.37, and 0.10–0.17 for between MZ, pooled DZ and siblings, and parent-offspring pairs respectively (Table 2). The variance component models considering polygenic effects only (AE model) was better fitted than the model further including the shared environment (ACE model). After the adjustment for age and sex, heritability was estimated to be 0.51 for HV-20, 0.69 for HV-15 and 0.47 for HVA, and age and sex accounted for 6.0% of total variance (Table 2).
TABLE 1 Overall Distribution of the Halux Valgus Cases and General Characteristics of Study Participants (n = 1,265)

1Figures in the square brackets indicate prevalence adjusted rates of HV standardized for age and sex. All other unspecified figures are crude mean and crude prevalence of HV; a Group mean values of HV angle showing statistically significant differences by ANOVA or t test (p < .05); b Group mean values of HV angle showing marked statistically significant differences by ANOVA, chi-square test or t-test (p < .001); SD, standard deviation; HV, hallux valgus (case or not); HVA, hallux valgus angle (as continuous measure); BMI, Quatalet's body mass index. Figures in bold indicate statistically significant findings.
TABLE 2 Intra-Class Correlations and Heritability Estimates of Hallux Valgus Status and Hallux Valgus Angle

Results of the best fitting models are indicated in bold type. CI, confidence interval; *Best fitting models in terms of AIC; MZ, monozygotic; DZ, dizygotic; A, additive genetics; C, shared environments; E, unique (unshared) environments; AIC, Akaike information criterion; HVA, hallux valgus angle (as continuous measure); HV, hallux valgus (case or not).1Covariates include age, sex, age*sex, age2, age2*sex.
Discussion
In the current study of Korean twin and family members, we found that HV is a heritable trait. This finding supports the previous studies that suggested the role of genes in developing HV deformity (Hardy & Clapham, Reference Hardy and Clapham1951; Pique-Vidal et al., Reference Pique-Vidal, Sole and Antich2007), and heritability estimates in this study are largely consistent with previous studies, considering the difference in study settings. Hardy and Clapham (Reference Hardy and Clapham1951) reported that heritability of HV was 0.63, based on 250 clinical HV cases and their relatives. Because the relatives of clinical cases share gene pools that predispose an increased risk of HV, the heritability of 0.63 might be overestimated. A recent report by Hannan et al. (Reference Hannan, Menz, Jordan, Cupples, Cheng and Hsu2013), which adopted the criteria of HVA over 15 degrees as determining HV, demonstrated that the overall heritability in a US white population was 0.29, and heritability was increased to 0.45 when it was estimated for those aged between 39 and60 years. The same study showed that overall heritability of lesser toe deformity was estimated to be 0.54.
The heritability estimates in the Korean population were larger than that found by Hannan et al. (Reference Hannan, Menz, Jordan, Cupples, Cheng and Hsu2013). Considering that most of the study participants in the Korean study comprised those aged between age 39 and 60, the two findings are generally compatible in indicating the importance of genetic contributions to HV deformity. When we further analyzed our data using the same cut-off as was used by Hannan et al. (Reference Hannan, Menz, Jordan, Cupples, Cheng and Hsu2013; HVA >15 degrees), the heritability estimate increased to 0.63. There could be some possible explanations for this difference of between 0.45 and 0.63. First, the heritability estimate is a measure of relative importance between genetic and environmental influences. It is not clear, however, whether the present ethnic differences in the HV heritability are attributable to the environmental or genetic variations in two populations. Second, classical twin studies might result in over-estimation of heritability because they do not discriminate additive from non-additive genetic components. However, the Healthy Twin study involves extended family members as well, and it is not likely that the increased heritability arises from the non-additive genetic effects. Third, differences in the diagnostic process might have influenced the heritability levels. We conducted radiologic measures for all, while Hannan et al. diagnosed HV using clinical inspection and examinations. But, as Hannan et al. also stated, the subluxation of the 1st metatarsophalangeal joint would confound the accuracy of this visual inspection screening. It is not clear in which direction the possible misclassification had exerted its effect, if it existed.
Pique-Vidal et al. (Reference Pique-Vidal, Sole and Antich2007) studied HV inheritance in 350 patients, and reported that penetrance of HV was 0.56. Penetrance estimates measure different aspects of genetic effects from that which can be estimated by heritability, which makes direct comparison between two findings inappropriate. Both findings, however, indicate that genetic influence is important in HV development. In studies by Hardy and Clapham (Reference Hardy and Clapham1951) and Pique-Vidal et al. (Reference Pique-Vidal, Sole and Antich2007), although radiographs and foot prints were used for the diagnosis of HV cases, a questionnaire asking about the family history of ‘bunions’ was used for their relatives. Previous studies are subject to information errors in both directions because the HV patient could have inflated the presence of family history (selective recall), or possible under-estimation (memory decay). As we did not rely on the recall and applied gold standard radiologic measure for all family members, we believe our findings will provide more accurate data.
Involving twins in addition to conventional families can enrich the analysis, particularly in dissecting familial shared environments and polygenic effects. Our findings suggest that genetics play an important role, while shared environments within family might not have significant effects. Participants in this study were not ascertained from their health status, including foot conditions. If we are interested in the genetic contribution to the occurrence of HV in a general population, our findings will be more appropriate than previous studies involving clinical HV cases. HV prevalence increases with age and in women (Nix et al., Reference Nix, Smith and Vicenzino2010). However, prevalence could vary depending on the study design, diagnostic criteria such as presence of bunions and foot pain (Owoeye et al., Reference Owoeye, Akinbo, Aiyegbusi and Ogunsola2011), or differences in methods of radiologic assessment. Cho et al. (Reference Cho, Kim, Kwon and Kim2009) reported the prevalence of HV in a rural Korean middle-aged population in which 13.2% had more than 25 degrees of HVA by radiological examinations. Considering that Cho et al.'s study subjects were about 10 years older than ours, findings from both studies are compatible. It is well documented that results from twin studies are readily generalizable, except for a few rare twin-associated conditions such as cerebral palsy. The prevalence of HV (HVA >20, 16.4%) would be representative of general Korean population. Our study involved more than 1,200 individuals with various types of family relationships. We believe the estimates from our study are stable with less standard error, due to sizeable samples. Our findings indicate that about 55–65% of all HV occurrences can be explained by the genes and demographics. Efforts to specify genetic variants will be required as a next step to dissect the etiology of HV. The heritability, however, is an estimate of overall aggregated genetic effects on total phenotypic variance, so that our finding of more than moderate genetic influence does not guarantee a successful gene identification study as it is.
Our study has several limitations as well. First, we were unable to adjust for shoe wearing habits. In this study, we did not find significant shared environmental effects on HV, other than age and sex effects. If shoe wearing habits and preference are significant but not shared within family, the environmental effects could have been under-estimated in our study. Second, the definition of HV in this study was based on a HVA over 15 or 20 degrees, and prevalence and genetic influence of this study might not directly apply to clinical cases with more pain and severe manifestations. Third, it is not certain whether the findings in this study are directly compatible with other populations.
We found that genetic influence plays a major role in developing HV in a population-based twin family study. Conduct of further studies to identify relevant genetic variants is warranted.
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/thg.2014.10
Acknowledgments
This study was supported by a grant of the National Project for Personalized Genomic Medicine, Ministry for Health & Welfare, Republic of Korea (A111218-12-GM10), National Research Foundation of Korea (2011-220-E00006 and 2012K2A1A2032536), and the second stage of Brain Korea 21.








