Introduction
Cognition has been described as “a suite of interrelated conscious (and unconscious) mental activities, including: pre-attentional sensory gating; attention; learning and memory; problem solving, planning, reasoning and judgment; understanding, knowing and representing; creativity, intuition and insight; ‘spontaneous’ thought; introspection; as well as mental time travel, self-awareness and meta-cognition (thinking and knowledge about cognition).”Reference Millan, Agid and Brune 1 The range of cognitive impairments reported in psychiatric disorders is wide and redundant across conditions.Reference Millan, Agid and Brune 1 These alterations do not only aggravate the course of the disorders but also strongly compromise patients’ quality of life and recovery. For example, cognitive symptoms (eg, working memory impairments) in bipolar disorder (BD) or schizophrenia (SCZ) predict the development and the severity of psychotic symptoms, suggesting that they may participate in the development of the diseases.Reference Jenkins, Bodapati, Sharma and Rosen 2 In addition, symptom severity (ie, intrusive thoughts, nightmares, and flashbacks) in patients with posttraumatic stress disorder (PTSD) is significantly correlated with impairments in attention, learning, memory, executive function, and visuospatial attention.Reference Qureshi, Long and Bradshaw 3 Cognitive deficits in attention, verbal learning, and verbal memory predict poorer general and psychosocial functioning in BD patients and impair recovery in later stages of the disease.Reference Baune, Li and Beblo 4 , Reference Andreou and Bozikas 5 They also negatively affect employment status and occupational functioning in adults with mood disorders or SCZ.Reference Baune, Li and Beblo 4 , Reference Tse, Chan, Ng and Yatham 6 – Reference Baune and Renger 8 There is therefore a great need for consideration for cognitive function in remission and recovery processes in psychiatry, which is emphasized by elevated rates of cognitive impairments after remissionReference Kar and Jain 7 , Reference Rock, Roiser, Riedel and Blackwell 9 , Reference Soni, Singh, Shah and Bagotia 10 representing a risk factor for relapse.Reference Majer, Ising and Kunzel 11
A better knowledge of the biological mechanisms underlying cognitive dysfunctions in patients suffering from psychiatric disorders is needed, as they would represent potential therapeutic targets in the management of cognition across psychiatric conditions. Numerous studies have implicated inflammation in the development of psychiatric disorders. In particular, it has been suggested that inflammation might underlie core symptoms of the disorders, such as somatic symptoms (eg, fatigue, sleep disturbances, appetite disturbances).Reference Dooley, Kuhlman, Robles, Eisenberger, Craske and Bower 12 However, there is less evidence linking elevated inflammation to cognitive deficits across psychiatric disorders.Reference Dooley, Kuhlman, Robles, Eisenberger, Craske and Bower 12 Thus, the purpose of this review is to summarize evidence that supports a role for inflammatory processes in the establishment of cognitive impairments across major depressive disorder (MDD), BD, SCZ, and PTSD.
Biological underpinnings of cognitive function
Various brain areas and mechanisms participate in the regulation of cognitive function in physiological conditions. In particular, these mechanisms have been extensively described in the hippocampus for learning and memory, whereas the mechanisms underlying other cognitive processes remain understudied. Mechanisms underlying learning and memory processes in the hippocampus encompass changes in neurotransmission at the synapse, namely Hebbian synaptic plasticity (including long term potentiation [LTP] and long term depression [LTD]).Reference Citri and Malenka 13 Hippocampal LTP inhibition through blockade or knockout of N-methyl-D-aspartate (NMDA) receptors impairs spatial learning and memory,Reference Martin and Morris 14 whereas enhancing LTP improves learning and memory performances.Reference Perusini, Cajigas and Cohensedgh 15 In addition, mice exhibiting impaired LTD display behavioral flexibility deficits.Reference Dong, Bai and Wu 16 To compensate with prolonged activity changes driven by Hebbian synaptic plasticity, homeostatic mechanisms relying on post-synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors adjust average synaptic strength to bring the level of post-synaptic response into an appropriate range.Reference Fernandes and Carvalho 17 It has been suggested that homeostatic plasticity could be a fundamental mechanism for the dynamic process of memory, providing a link between early and late memory formation processes.Reference Tetzlaff, Kolodziejski, Timme, Tsodyks and Worgotter 18 Hippocampal Hebbian plasticity is also tightly interrelated with the formation of new neurons, namely neurogenesis,Reference Bruel-Jungerman, Davis, Rampon and Laroche 19 , Reference Nissant, Bardy, Katagiri, Murray and Lledo 20 which also contributes to learning and memory. Indeed, the rate of neurogenesis in rodents was found to be positively correlated to spatial learning and memory performances.Reference Hollands, Tobin and Hsu 21 , Reference Aarse, Herlitze and Manahan-Vaughan 22 In the hippocampus, these key regulatory mechanisms are dependent on the synthesis of neurotrophic factors such as brain-derived neurotrophic factor (BDNF)Reference Kino 23 – Reference Aarse, Herlitze and Manahan-Vaughan 26 and the activation of various pathways such as the HPA axisReference Kino 27 – Reference Pocivavsek, Wu, Potter, Elmer, Pellicciari and Schwarcz 31 and the kynurenine pathway, which are therefore able to modulate learning and memory.Reference de Quervain, Schwabe and Roozendaal 32 – Reference Pocivavsek, Wu, Elmer, Bruno and Schwarcz 36
Inflammation and cognitive function in physiological conditions: mechanisms
The relationship between raised levels of inflammation and cognitive changes across inflammation-associated conditions (eg, obesity, rheumatoid arthritis, HIV infection)Reference Castanon, Lasselin and Capuron 37 – Reference Saylor, Dickens and Sacktor 39 has suggested a role for inflammation in the regulation of cognition in physiological conditions. The role of peripheral and brain inflammatory mediators (ie, cytokines) in influencing learning and memory was previously reviewed under the name of “cytokine model of cognitive function.”Reference McAfoose and Baune 40 Microglia, the immune cells of the brain, do not only play a role in brain immune function but are also strong regulators of neurological functionReference Singhal and Baune 41 (see Figure 1) and cognition in physiological conditions. Indeed, microglia depletion or inhibition in mice negatively impairs learning and memory.Reference Torres, Danver and Ji 42 Although microglia can directly modulate cognition, it is noteworthy that they can also perform this role by secreting inflammatory mediators such as cytokines. As previously reviewed, inflammatory cytokines (ie, interleukin-1β [IL-1β], IL-6, and tumor necrosis factor-α [TNF-α]) are required for the physiological regulation of memory processes since disrupting their signaling pathway leads to decreased learning and memory.Reference Baune, Wiede, Braun, Golledge, Arolt and Koerner 43 – Reference Hryniewicz, Bialuk, Kamiński and Winnicka 46 However, the regulatory role of these cytokines on cognition is dose-dependent since overexpression of IL-1β or TNF-α disrupts normal learning and memory in rodents.Reference Barrientos, Higgins, Sprunger, Watkins, Rudy and Maier 47 , Reference Fiore, Angelucci, Alleva, Branchi, Probert and Aloe 48
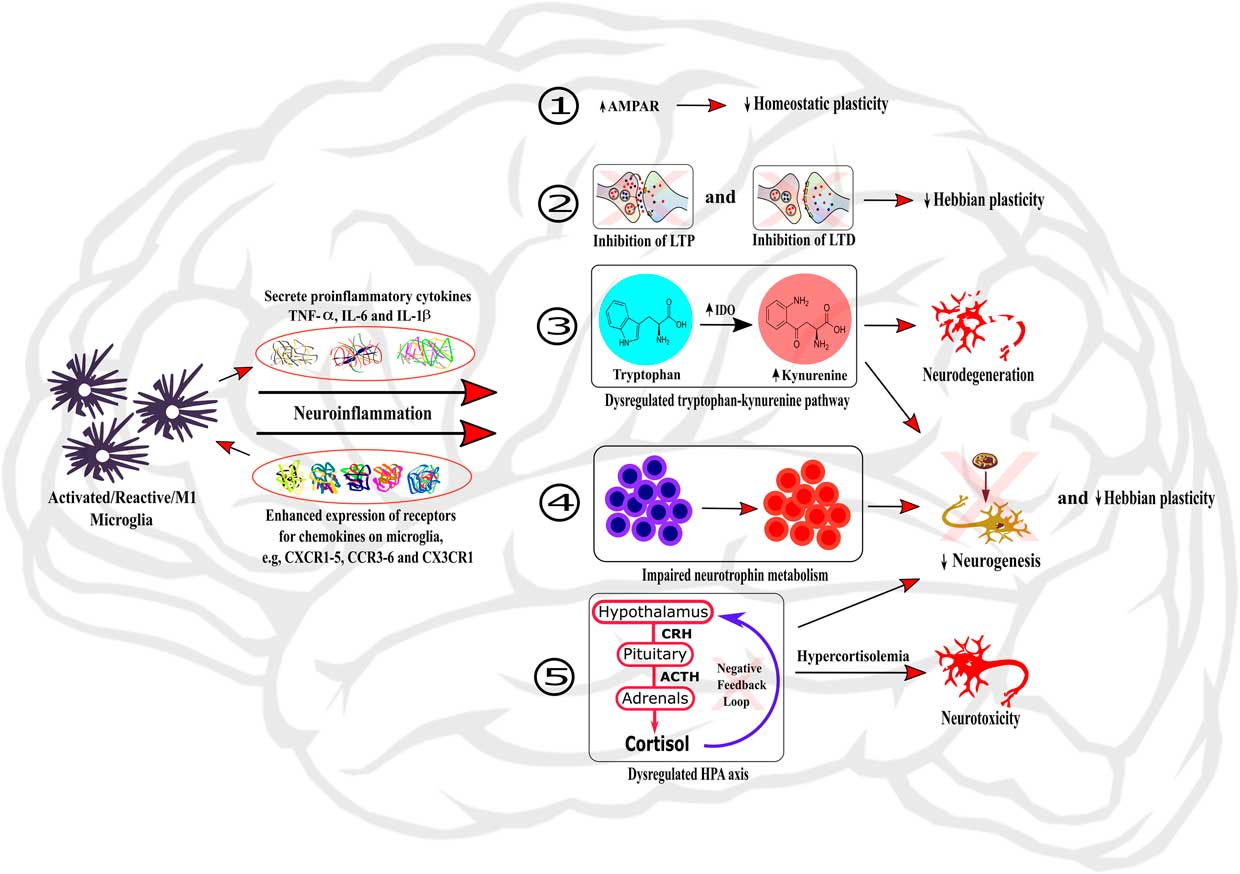
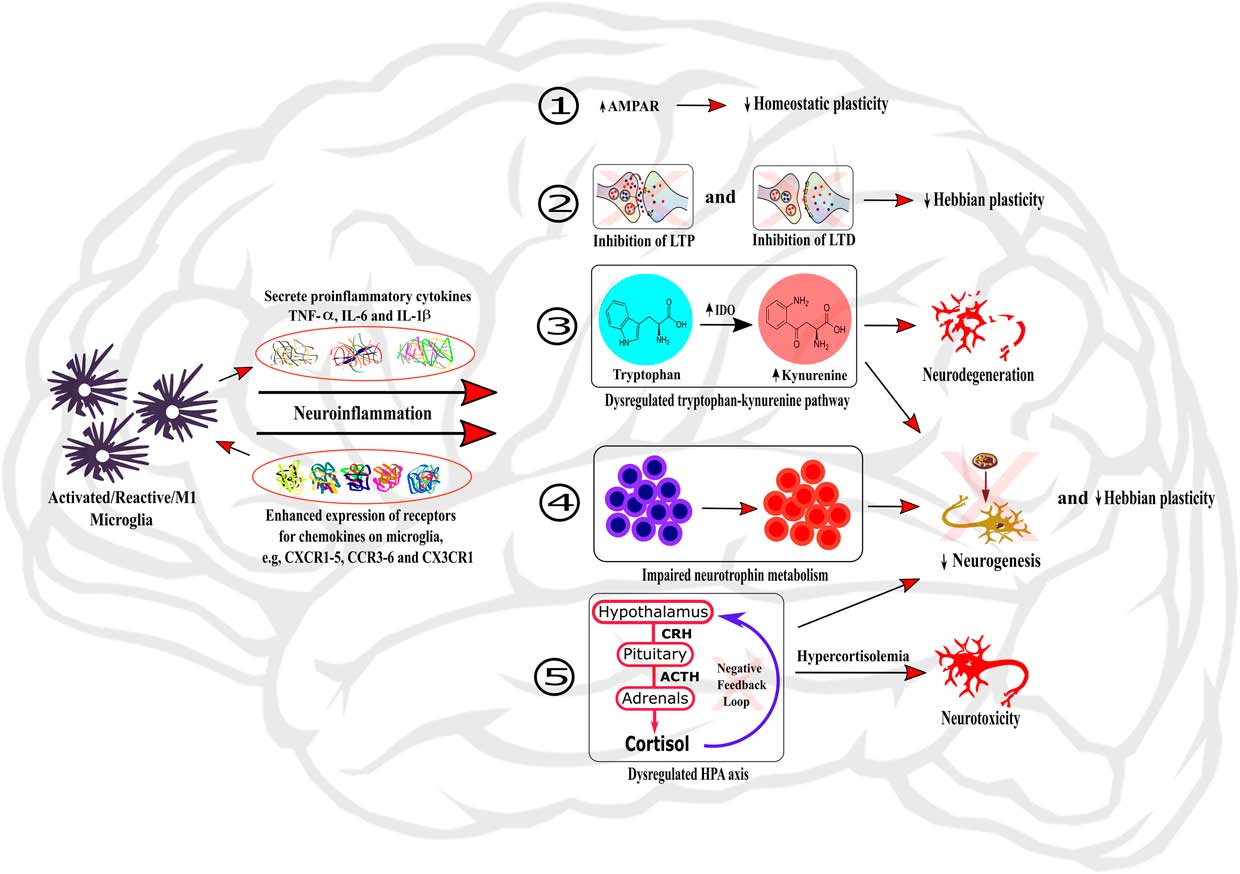
Figure 1 Role of neuroinflammation in the dysregulation of neurobiological processes underlying cognition. The activation of microglia within the brain induces neuroinflammation through the secretion of local pro-inflammatory cytokines and the enhanced expression of chemokine receptors on microglia. Neuroinflammation can then induce dysregulations of neurobiological mechanisms regulating cognitive processes by: (1) changing the expression and activity of AMPAR, therefore impairing homeostatic plasticity; (2) inhibiting LTP and LTD processes and hence impairing Hebbian plasticity; (3) dysregulating the tryptophan-kynurenine pathway, subsequently causing neurodegeneration; (4) impairing neurotrophin metabolism; and (5) dysregulating HPA axis, leading to hypercortisolemia and subsequent neurotoxicity. In addition, the processes (3), (4), and (5) also participate to impairment of neurogenesis and Hebbian plasticity processes.
Recent evidence suggests that disruption of microglia activation alters hippocampus-dependent neuronal plasticity and learning and memory performance in adulthood.Reference Maggi, Scianni, Branchi, D’Andrea, Lauro and Limatola 49 , Reference Rogers, Morganti and Bachstetter 50 Microglial processes continuously interact with synapses in a glutamate-dependent way,Reference Eyo, Peng, Swiatkowski, Mukherjee, Bispo and Wu 51 suggesting a role in learning and memory through their impact on synaptic plasticity. In addition, microglia indirectly modulate synaptic plasticity through the production of inflammatory cytokines.Reference Pickering and O’Connor 52 IL-1β and TNF-α are critical in the establishment of synaptic plasticity since their knockout induces impaired LTPReference Avital, Goshen and Kamsler 53 and LTD,Reference Albensi and Mattson 54 respectively. Glial-derived TNF-α also strongly regulates homeostatic plasticity by inducing exocytosis of AMPA receptors and inhibiting astrocyte glutamatergic transporters at the synapse.Reference Stellwagen, Beattie, Seo and Malenka 55 – Reference Beattie, Stellwagen and Morishita 57 However, the effect of inflammatory cytokines on synaptic plasticity often follows an inverted U-shape since supra-physiological doses of IL-1β, IL-6, and TNF-α disrupts normal LTP,Reference Murray and Lynch 58 – Reference Cumiskey, Butler, Moynagh and O’Connor 60 possibly linking raised inflammation to cognitive impairments. Similarly to what has been reported for pro-inflammatory cytokines, anti-inflammatory cytokines, such as IL-10, also participate in the regulation of hippocampal synaptic plasticity in a dose-dependent manner.Reference Kelly, Lynch and Vereker 61 – Reference Almolda, de Labra and Barrera 63 Microglia and pro- and anti-inflammatory cytokines do not only regulate Hebbian and homeostatic plasticity but can also influence brain function through their effects on neurogenesis. During development, microglia coordinate synaptic pruning, ie, the elimination of weak synapses in order to maintain and strengthen functional synapses.Reference Hua and Smith 64 During adulthood, neurogenesis is then highly dependent on the crosstalk between microglia and neurons through the CX3C chemokine receptor 1/CX3C chemokine ligand 1 (CX3CR1/CX3CL1) pathway.Reference Delpech, Madore, Nadjar, Joffre, Wohleb and Laye 65 , Reference de Miranda, Zhang, Katsumoto and Teixeira 66 Cytokines such as IL-1β and IL-6 have a dual role on adult neurogenesis in the hippocampus. On one hand, they exert a critical role in the establishment of neurogenesis.Reference Meng, Zhang, Shi, Zhang and Yuan 67 , Reference Spulber, Oprica, Bartfai, Winblad and Schultzberg 68 On the other hand, their overexpression in the brain negatively affects adult neurogenesis.Reference Vallieres, Campbell, Gage and Sawchenko 69 , Reference Wu, Hein, Moravan, Shaftel, Olschowka and O’Banion 70 Similarly, TNF-α may exert a dual role on adult neurogenesis, through a differential effect of its receptors TNF-R1 and TNF-R2,Reference Chen and Palmer 71 although the underlying mechanisms remain understudied. Recently, IL-10 was also described as an enhancer of postnatal neurogenesis.Reference Pereira, Font-Nieves, Van den Haute, Baekelandt, Planas and Pozas 72
Microglia and inflammatory mediators may also have an indirect effect on cognition-associated biological mechanisms through modulation of neurotrophic factors levels and signaling pathway activation. Although the link between inflammation, cognition, and neurotrophic factors needs further consideration, there is evidence that cytokines can modulate BDNF levels and activity.Reference Calabrese, Rossetti, Racagni, Gass, Riva and Molteni 73 In particular, immune stimulation decreases brain BDNF expression and activity,Reference Gonzalez, Machado and Vilcaes 74 , Reference Tong, Prieto and Kramar 75 therefore altering synaptic plasticity in the hippocampus.Reference Tong, Prieto and Kramar 75 In mice, BDNF removal from microglia revealed that these cells regulate memory by promoting synapse formation through BDNF signaling.Reference Parkhurst, Yang and Ninan 76 Along with altering neurotrophic factor activity, cytokine signaling pathways can interact with GC receptor signaling and therefore change GC action.Reference Pace, Hu and Miller 77 Inflammatory cytokines can indeed influence the production of all the hormones produced along the hypothalamic–pituitary–adrenal (HPA) axisReference Silverman and Sternberg 78 and modulate GR function at multiple levels, from expression to translocation and associated signaling pathways.Reference Pace and Miller 79 In addition to their effects on neurotrophic factors and the HPA axis, inflammatory processes can influence kynurenine pathway activation. Pro-inflammatory cytokines induce hippocampal activation of the kynurenine-producing enzyme indoleamine 2,3-dioxygenase (IDO),Reference Andre, O’Connor, Kelley, Lestage, Dantzer and Castanon 80 , Reference O’Connor, Andre and Wang 81 which participates in the regulation of learning and memory.Reference Heisler and O’Connor 35 , Reference Too, Li and Suarna 82
Inflammation and Cognition Across Psychiatric Conditions
The key role that inflammatory processes play in the regulation of the neurobiological processes underlying cognition in physiological conditions suggests that dysregulations of the immune system could participate in cognitive alterations reported across psychiatric diseases. In agreement with this hypothesis, alterations of neurobiological mechanisms regulating cognition are redundant across disorders (see Table 1). Here, we review the evidence suggesting that inflammatory processes, in particular activated microglia and inflammatory cytokines, play a role in impaired cognitive performance associated with psychiatric disorders through the regulation of the neurobiological processes underlying cognitive function (see Figure 1).
Table 1 Main biological mechanisms impairments across psychiatric conditions
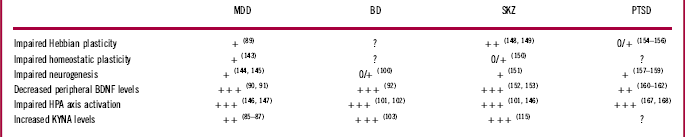
0: essentially absent; 0/+: anecdotal, poorly documented, ambiguous; +: preclinical; ++: clinical; +++: clinical, consistent; ?: not clearly evaluated.
Reference numbers are in parentheses.
Inflammation and cognition in major depressive disorder
Levels of inflammatory markers have been found to be associated with cognitive performance in MDD. In patients with MDD, elevated serum levels of TNF-α, TNF-R1, and TNF-R2 negatively correlate with performance in executive functioning, attention, learning, working, and declarative memories.Reference Bobinska, Galecka, Szemraj, Galecki and Talarowska 83 Similarly, elevated plasma levels of C-reactive protein (CRP) and IL-6 are associated with impaired cognitive performance in the domains of attention and executive function and of verbal memory and psychomotor speed, respectively.Reference Chang, Lee and Gean 84 – Reference Goldsmith, Haroon and Woolwine 86 It is noteworthy that CRP and IL-6 levels are not only associated with cognitive symptoms of depression at baseline but also predict those symptoms at 12 years follow-up, suggesting that inflammation contributes to the progression of MDD rather than to the later stages of the disease.Reference Gimeno, Kivimaki and Brunner 87 This relationship appears to be unilateral since cognitive symptoms of depression at baseline are not predictive of inflammatory status at follow-up. Although double-blind, randomized, placebo-controlled trials with anti-inflammatory agents are necessary to establish a causal link between inflammation and cognition in MDD, acute treatment with the cyclooxygenase (COX)-2 inhibitor Celecoxib has been reported to improve cognitive function in an elderly depressed woman with recurrent MDD.Reference Chen, Tzeng and Chen 88
It is noteworthy that individuals with MDD display dysregulations of the kynurenine (KYNA) pathway, which could mediate the relationship between elevated levels of inflammatory markers and cognitive function through its effects on brain plasticity. Elevated levels of inflammatory markers were found to be associated with decreased urinary KYNA in MDD patients.Reference Peacock, Scheiderer and Kellermann 89 Interestingly, KYNA/3-hydroxykynurenine (3-HK) was also reported to negatively correlate with hippocampal activity during memory recall, and KYNA/quinolinic acid (QA) correlated with negative specific memory recall and with hippocampal and amygdala volume in MDD patients.Reference Young, Drevets, Dantzer, Teague, Bodurka and Savitz 90 , Reference Savitz, Drevets and Smith 91 It might be hypothesized that the kynurenine pathway could participate in the impairment of cognitive functions observed in MDD by influencing glutamatergic transmission in brain structures associated with cognitive processes. Numerous alterations in glutamatergic synaptic plasticity have indeed been reported in animal models of depression.Reference Mahati, Bhagya, Christofer, Sneha and Shankaranarayana Rao 92 Inhibition of microglia activation prevents impairments of both spatial memory and hippocampal LTP in a rodent model of depression. This effect is likely to involve a role of GluR1 phosphorylation.Reference Liu, Li and Dai 93 BDNF is also likely to be a mediator of inflammation-associated cognitive impairments in MDD. In cancer patients with depression, plasma IL-6 levels predict serum BDNF levels, which are significantly associated with short-term memory performance.Reference Jehn, Becker and Flath 94 In addition, inhibiting TNF-α in a rat model of depression prevents stress-induced cognitive impairments as well as the associated reduction of hippocampal BDNF expression.Reference Sahin, Karson, Balci, Yazir, Bayramgurler and Utkan 95
Inflammation and cognition in bipolar disorder
Currently, few studies have reported associations between inflammatory processes and cognitive performances in BD.Reference Bauer, Pascoe, Wollenhaupt-Aguiar, Kapczinski and Soares 96 In individuals with BD, plasma CRP levels are negatively correlated with immediate memory, language, and attention.Reference Dickerson, Stallings, Origoni, Vaughan, Khushalani and Yolken 97 Similarly, elevated levels of IL-1ra and TNF-α are associated with worse memory performances, even during euthymic states.Reference Hope, Hoseth and Dieset 98 , Reference Doganavsargil-Baysal, Cinemre and Aksoy 99 Elevated plasma levels of soluble TNF (sTNF)-RI were also found to be associated with impaired declarative memory in patients with BD.Reference Hoseth, Westlye and Hope 100 The only study assessing the relationship between cerebrospinal fluid (CSF) inflammatory markers and cognition in BD reported a negative association between CSF concentration of the inflammatory biomarker YLK-40 and executive function in those patients.Reference Rolstad, Jakobsson and Sellgren 101 It is noteworthy that in addition to elevated peripheral inflammation reported in BD patients with cognitive impairments, increased microglial activation was reported in the right hippocampus of BD patients in comparison to healthy controls.Reference Haarman, Riemersma-Van der Lek and de Groot 102 Hence, these studies provide evidence that elevated inflammatory profile is negatively associated with cognitive function in BD.
Various neurobiological systems have been reported to potentially participate in inflammation-associated cognitive impairments in BD. A recent study reported that pro-inflammatory cytokines such as TNF-α decrease white matter integrity in BD individuals,Reference Benedetti, Poletti and Hoogenboezem 103 which could be mediated by alterations in neurogenesis.Reference Czeh and Lucassen 104 Cytokines may also alter cognition by influencing the activity of the HPA axis, subsequently leading to impaired neuroplasticity. Indeed, HPA axis alterations are associated with impaired cognition in individuals with BD. GR insensitivity was reported in BD,Reference Fries, Vasconcelos-Moreno and Gubert 105 and mifepristone (GR antagonist) treatment for 1 week improves spatial working memory performance in BD.Reference Watson, Gallagher and Porter 106 In addition, a very recent study showed that adult males with BD display elevated plasma 3-HK/KYNA ratio, which is significantly associated with poorer declarative memory performances.Reference Platzer, Dalkner and Fellendorf 107 Given the role that inflammatory cytokines play in the regulation of the IDO pathway, inflammation may dysregulate this pathway, leading to an imbalance between neuroprotective and neurotoxic metabolites and to a subsequent cognitive impairment in BD patients. Finally, it is worth mentioning that peripheral BDNF levels, which can also be regulated by inflammation, predict cognitive function in BD. Moreover, the BDNF val66met polymorphism could be a risk factor for cognitive impairment in this disease,Reference Bauer, Pascoe, Wollenhaupt-Aguiar, Kapczinski and Soares 96 further reinforcing the possible role of BDNF in mediating the effects of inflammation on cognition in BD.
Inflammation and cognition in schizophrenia
Inflammation has also been extensively reported to be a potential player in the etiology and pathophysiology of SCZ. Epidemiological studies have reported elevated risk of schizophrenia following prenatal or childhood exposure to infection. Infection then mediates peripheral and central inflammatory responses which in turn alter brain development (for a review, see MeyerReference Meyer 108 ). Similarly to what has been reported in other psychiatric conditions, cognitive impairments associated with schizophrenia are correlated with raised peripheral inflammation. A recent systemic review conducted on SCZ patients reported an association between plasma CRP levels and worse cognitive performance including in the domains of attention, memory, and learning abilities.Reference Misiak, Stanczykiewicz, Kotowicz, Rybakowski, Samochowiec and Frydecka 109 Similarly, significant negative associations have been reported between general cognitive function and serum IL-6, sTNF-R1, and IL-1ra levels,Reference Hope, Hoseth and Dieset 98 , Reference Frydecka, Misiak and Pawlak-Adamska 110 and elevated peripheral IL-1β mRNA levels are associated with both impairments in verbal fluency and brain volume reduction in a subgroup of patients with SCZ.Reference Fillman, Weickert and Lenroot 111 It is noteworthy that an association has also been reported between cognitive impairments and anti-inflammatory cytokines in SCZ, since serum IL-10 levels negatively correlate with cognitive factor (made up of 3 items of the Positive and Negative Syndrome Scale).Reference Xiu, Tian and Chen 112 , Reference Xiu, Yang and Tan 113 Particularly, patients who carry the AA allele of the IL10-592 A/C polymorphism perform worse in attention, suggesting that this IL-10 allele could contribute to cognitive impairments in SCZ.Reference Xiu, Tian and Chen 112 Moreover, inflammatory pathways are enriched in mutations associated with cognitive impairments in SCZ patients.Reference Fischer and Drago 114 In addition to these studies showing association between inflammation and cognition in SCZ, Müller et al Reference Müller, Riedel, Schwarz and Engel 115 reported that decreasing inflammation through anti-inflammatory add-on to risperidone treatment for 5 weeks trends to improve cognition factor in SCZ patients (F1,47=3.64; p=0.06).
A longitudinal, double-blind, randomized, placebo-controlled study showed that minocycline add-on to atypical antipsychotic treatment has a beneficial effect on executive functioning (such as working memory, cognitive shifting, and cognitive planning), suggesting that inhibiting microglia activation in patients with SCZ could be a strategy to decrease SCZ-associated cognitive impairments.Reference Levkovitz, Mendlovich and Riwkes 116 This is in agreement with the microglia hypothesis of SCZ, which states that the neuropathology of SCZ is closely associated with elevated microglia activation.Reference Monji, Kato and Kanba 117 , Reference Laskaris, Di Biase and Everall 118 Indeed, inflammatory cytokines and free radicals produced by activated microglia in animal models of SCZ lead to decreased neurogenesis, white matter abnormalities, and neuronal degeneration, which may participate in the pathophysiology of the disease.
Similarly to what has been suggested for MDD and BD, inflammation-mediated kynurenine pathway dysfunctions could also participate in cognitive alterations in SCZ, since patients display raised levels of KYNA in the CSF.Reference Linderholm, Skogh and Olsson 119 This effect could be mediated by changes in glutamatergic neurotransmission. MüllerReference Müller 120 suggested that elevated inflammation in SCZ may promote the production of the NMDA antagonist KYNA, therefore resulting in a glutamatergic imbalance. The glutamate hypothesis of SCZ suggests that a deficit in glutamatergic transmission in the brain of SCZ patients may lead to dopaminergic system dysfunction, which may in turn exacerbate glutamatergic transmission impairments, eventually leading to psychotic and cognitive symptoms.Reference Müller 120 Decreased glutamatergic neurotransmission may be associated with alterations in synaptic vesicles transportation, which is linked to IL-10 levels in the brain of SCZ patients.Reference Xiu, Tian and Chen 112
Inflammation and cognition in PTSD
Currently, the link between inflammatory processes and cognitive impairments remains understudied in the context of PTSD. However, a few recent studies reported elevated levels of inflammatory markers (such as CRP, TNF-α, IL-6, and IL-1β) in the plasma of individuals with a diagnosis of PTSD.Reference Passos, Vasconcelos-Moreno and Costa 121 – Reference Miller, Driscoll, Smith and Ramaswamy 123 In a rat model of PTSD, chronic consumption of curcumin, a component with anti-inflammatory properties, impairs the consolidation and reconsolidation of fear memories, suggesting a possible role for inflammation in memory processes.Reference Monsey, Gerhard, Boyle, Briones, Seligsohn and Schafe 124 Interestingly, plasma levels of sTNF-RII are associated with reduced hippocampal volume in Gulf War veterans with current and past PTSD.Reference O’Donovan, Chao and Paulson 125 Hence, one can hypothesize that inflammation could impair cognition by reducing hippocampal volume via its deleterious effect on neurogenesis or HPA axis activation.Reference Boku, Nakagawa, Toda and Hishimoto 126 In agreement with this last assumption, the GC receptor antagonist mifepristone improves cognition in Gulf War veterans with PTSD.Reference Golier, Caramanica and Michaelides 127
Future Directions
Anti-inflammatory strategies to improve cognition across psychiatric conditions
The redundant association between inflammatory alterations and cognitive processes across psychiatric disorders suggests that deconstructing psychiatric disorders to account for the heterogeneity across individuals and consider patients’ endophenotypes may be promising in the development of novel strategies in the management of cognitive alterations in psychiatry. A cross-disorder approach is in agreement with the Research Domain Criteria (RDoC) initiative led by the National Institute of Mental Health (NIH), which is aimed at developing new ways of classifying mental disorders based on dimensions of both observable behavior and neurobiological measures, in order to develop new and individualized treatments. The evidence reviewed here suggests that the use of anti-inflammatory strategies as adjunctive therapies across diagnoses could improve the management of cognitive impairments in psychiatry.
Pharmacological approaches
Nonsteroidal anti-inflammatory drugs (NSAIDs) such as celecoxib (COX-2 inhibitor) are widely used in the treatment of inflammatory conditions (eg, arthritis, multiple sclerosis). In the last decades, they have also been used as effective additional therapies to antidepressant and antipsychotic treatments across psychiatric conditions.Reference Müller, Riedel and Schwarz 128 – Reference Kohler, Benros and Nordentoft 130 However, only a few preliminary data suggest a possible enhancing effect of the anti-inflammatory medication celecoxib on cognition in psychiatric disorders.Reference Chen, Tzeng and Chen 88 , Reference Müller, Riedel, Schwarz and Engel 115 Interestingly, NSAIDs were shown to improve memory in mouse models of neurotoxicityReference Syed, Ikram, Yaqinuddin and Ahmed 131 and Parkinson’s disease,Reference Naeem, Ikram, Khan and Rao 132 suggesting that their impact on cognition across psychiatric conditions requires further examination.
Nutritional interventions
Numerous research efforts have reported a beneficial effect of nutrients such as n-3 polyunsaturated fatty acids (n-3 PUFAs) and flavonoids on inflammatory processes underlying cognitive dysfunctions.Reference Vauzour, Martinsen and Laye 133 Indeed, these nutrients and their metabolites have been extensively described to potently reduce microglia activation and cytokine production in inflammatory conditions and improve associated alterations in HPA axis activation, synaptic plasticity, and BDNF production.Reference Vauzour, Martinsen and Laye 133 – Reference Rey, Nadjar and Buaud 136 It is noteworthy that polyphenols (such as flavonoids) and n-3 PUFAs can alleviate cognitive impairments in inflammation-associated conditions such as aging and disorders such as Parkinson’s disease and Alzheimer’s disease.Reference Vauzour, Martinsen and Laye 133 , Reference Bensalem, Servant and Alfos 137 There is also evidence that they have a beneficial effect on psychiatric disorders,Reference Müller, Riedel and Schwarz 128 although it is unclear whether those cognitive improvements are mediated by inflammatory changes.
Physical activity
Physical activity has been reported to decrease inflammation in both preclinical and clinical models of psychiatric conditions such as MDD.Reference Eyre and Baune 138 , Reference Gleeson, Bishop, Stensel, Lindley, Mastana and Nimmo 139 In rodents, exercise does not only enhance anti-inflammatory processes at the cellular level, by changing microglial phenotype, but also at the molecular level by increasing the production of anti-inflammatory cytokines in the brain.Reference Eyre and Baune 138 This anti-inflammatory effect of exercise positively influences the activity of the HPA axis as well as neuronal proliferation, neurotrophic factor levels, and the activation of the kynurenine pathway,Reference Eyre and Baune 138 which suggests that it could positively impact cognition across psychiatric conditions. In agreement with this hypothesis, moderate physical activity has a beneficial effect on cognition in patients with psychiatric conditions.Reference Jahshan, Rassovsky and Green 140 – Reference Greer, Furman and Trivedi 142
Conclusion
The numerous cognitive impairments across major psychiatric disorders highlight a strong need for consideration since they not only affect the quality of life but also the treatment and recovery of patients. However, the mechanisms underlying these deficits are still understudied and must be addressed to allow a better management of the disorders. The common pattern of cognitive impairments across psychiatric conditions suggests shared mechanisms potentially leading to their causation. As described in this review, inflammation could be a shared mechanism underlying the development of cognitive impairments in MDD, BD, SCZ, and PTSD. Indeed, raised inflammatory processes (ie, activated microglia and elevated levels of inflammatory cytokines) can disrupt neurobiological mechanisms regulating cognitive processes (see Figure 2). However, although most studies report associations or correlations between inflammatory biomarkers, cognitive-related biological mechanisms, and cognitive performance, causal evidence is still strongly lacking. Few studies have evaluated the role of inflammation in cognitive alterations in other psychiatric conditions such as autism or attention deficit hyperactivity disorder, but one may hypothesize that the underlying mechanisms could be similar given the shared pattern of cognitive alteration across psychiatric conditions.Reference Millan, Agid and Brune 1
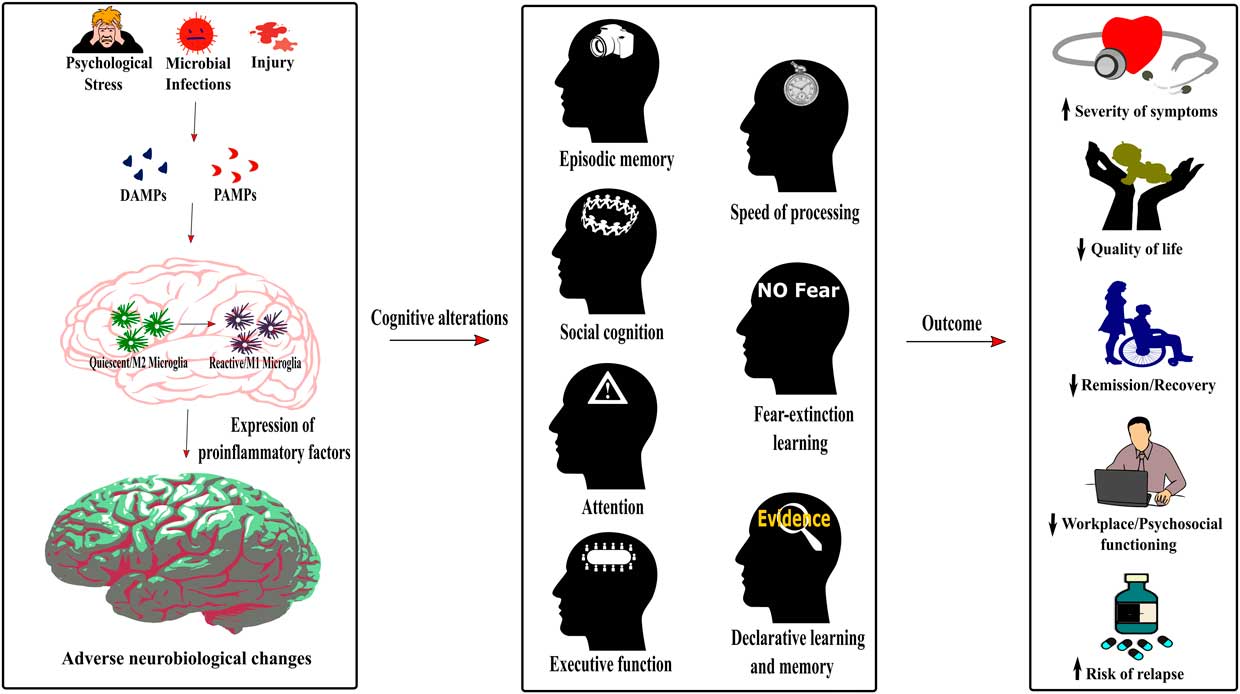
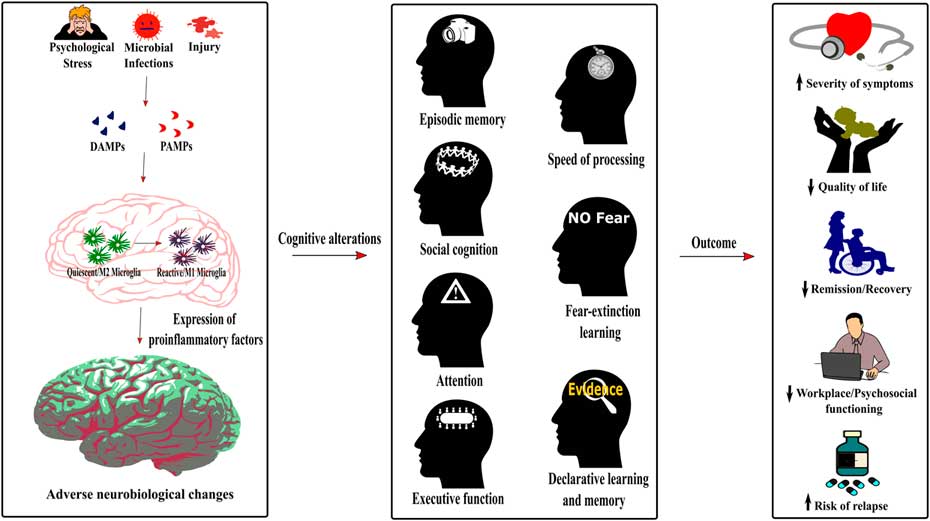
Figure 2 Role of neuroinflammation in the cognitive alterations and disease outcomes across psychiatric conditions. The detection of damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs) by the immune system following psychological stress, infections or injury induces a switch of the microglia phenotype from a quiescent M2 phenotype to an activated or reactive M1 phenotype. As described in Figure 1, this induces neuroinflammation, therefore dysregulating neurobiological processes underlying cognition. The cognitive alterations resulting from this inflammatory state are redundant across psychiatric conditions and encompass impaired episodic memory, processing speed, social cognition, fear-extinction learning, attention, executive function, and declarative learning and memory. Importantly, this common pattern of cognitive alterations in psychiatric disorders increases the severity of symptoms, decreases the quality of life and workplace and psychosocial functioning of patients, has a negative effect on remission and recovery processes, and increases the risk of relapse.