Introduction
The early life environment is a major contributor to an individual's developmental trajectory (Shonkoff et al., Reference Shonkoff, Garner, Siegel, Dobbins, Earls and Wood2012). Chronic stress exposure in early life, referred to in the literature as early life stress (ELS) or toxic stress, increases the risk of adverse behavioral, physical, and mental outcomes throughout life (Shonkoff et al., Reference Shonkoff, Garner, Siegel, Dobbins, Earls and Wood2012). Indeed, ELS in the form of abuse (sexual, physical, and emotional), neglect (physical and emotional), and parental loss has been associated with several health outcomes in children and adults, such as poor social and academic competences, inflammatory and cardiovascular disorders, substance abuse, sleep disorders, posttraumatic stress disorders (PTSD), bipolar disorders (BP), major depression (MDD), and attempted suicide (Bick & Nelson, Reference Bick and Nelson2016; Danese, Pariante, Caspi, Taylor, & Poulton, Reference Danese, Pariante, Caspi, Taylor and Poulton2007; Nemeroff, Reference Nemeroff2016; Shonkoff, Reference Shonkoff2016).
One possible mechanism underlying the pathogenic nature of ELS is through modifications of physiological systems responding to stress, such as the hypothalamus–pituitary–adrenal (HPA) axis (Heim, Reference Heim, Gellman and Turner2013). The HPA axis plays a key role for adequate responding to, coping with, and subsequent recovery from environmental threats that disrupt homeostasis (McEwen, Reference McEwen2007; van Bodegom, Homberg, & Henckens, Reference van Bodegom, Homberg and Henckens2017). The exposure to a stressor activates a cascade of central and peripheral events that causes the release of cortisol from the adrenal cortex (van Bodegom et al., Reference van Bodegom, Homberg and Henckens2017). Cortisol promotes physiological and behavioral changes as part of the fight-or-flight response, which allows the organism to deal with the stressor (van Bodegom et al., Reference van Bodegom, Homberg and Henckens2017). During the acute phase of the HPA axis stress response, the binding of cortisol to the glucocorticoid receptor (GR) induces a negative feedback that brings cortisol back to prestress levels, contributing to the stress response being terminated (Stephens & Wand, Reference Stephens and Wand2012). However, chronic stress exposure during development causes the HPA axis to adapt, in order to optimize stress responses to future stressors and promote survival of the individual within its environment (McEwen, Reference McEwen2004). For instance, most of the studies with children exposed to ELS have demonstrated altered HPA axis stress responses and higher levels of circulating cortisol, as well as other stress mediators (Agorastos, Pervanidou, Chrousos, & Baker, Reference Agorastos, Pervanidou, Chrousos and Baker2019). Because the effects of cortisol include threat vigilance, heightened emotional arousal, and motivation for self-defense, increased cortisol levels may provide some advantages in stressful environments (Thompson, Reference Thompson2014). However, the allocation of resources devoted to deal with stressful conditions presents several trade-offs, such as immune function suppression, enhanced cardiovascular tone, and diminished ability to concentrate, remember things, control thoughts, and regulate emotions (Thompson, Reference Thompson2014). These factors and the long-term adaptations of the HPA axis to ELS contribute to lay a foundation for lifelong adverse outcomes (Thompson, Reference Thompson2014; Shonkoff et al., Reference Shonkoff, Garner, Siegel, Dobbins, Earls and Wood2012; van Bodegom et al., Reference van Bodegom, Homberg and Henckens2017).
A growing body of evidence suggests that the maintenance of HPA axis adaptations to ELS is caused by long-lasting molecular modifications of the gene coding for the GR (Turecki & Meaney, Reference Turecki and Meaney2016). These modifications involve epigenetic mechanisms, which affect gene expression without altering the underlying DNA sequence, and are both mitotically heritable and reversible (Tammen, Friso, & Choi, Reference Tammen, Friso and Choi2013). DNA methylation (DNAm), the most studied and well-characterized epigenetic modification, regulates gene expression through the addition of methyl groups at cytosines in cytosine–guanine dinucleotides (CpG) (Kumar, Chinnusamy, & Mohapatra, Reference Kumar, Chinnusamy and Mohapatra2018; Portela & Esteller, Reference Portela and Esteller2010). Higher levels of methylation decrease the binding of transcription factors to the DNA, causing gene silencing and decreased gene expression (Kumar et al., Reference Kumar, Chinnusamy and Mohapatra2018; Portela & Esteller, Reference Portela and Esteller2010). Hypermethylation of the GR gene (NR3C1) in the hypothalamus decreases levels of hypothalamic GR (Labonte et al., Reference Labonte, Yerko, Gross, Mechawar, Meaney, Szyf and Turecki2012). This contributes to suppression of HPA axis negative feedback and normal termination of the stress response (Labonte et al., Reference Labonte, Yerko, Gross, Mechawar, Meaney, Szyf and Turecki2012; Liu & Nusslock, Reference Liu and Nusslock2018).
The majority of studies in rodents and humans (children and adults) indicates that ELS is linked to NR3C1 hypermethylation, decreased levels of GR, disrupted HPA axis negative feedback, and abnormal physiological responses, such as increased or decreased cortisol reactivity in response to stressors (Parent et al., Reference Parent, Parade, Laumann, Ridout, Yang, Marsit and Tyrka2017; Turecki & Meaney, Reference Turecki and Meaney2016).
The precise mechanisms through which ELS modifies epigenetic patterns are still unclear (Nagy, Vaillancourt, & Turecki, Reference Nagy, Vaillancourt and Turecki2018). However, studies in rodents identified a molecular pathway explaining how maternal behavior alters epigenetic patterns of the NR3C1. Maternal licking/grooming behavior induces increased hippocampal serotonergic tone, subsequent activation of the nerve growth factor, and recruitment of enzymes to the NR3C1 promoter, such as histone acetyltransferases and DNA demethylases, which lead to increased hypothalamic GR in the offspring (Weaver, Reference Weaver2007). Among methylation studies in children, ELS in the form of maltreatment and exposure to maternal depression has been repeatedly associated with increased NR3C1 methylation (Cicchetti & Handley, Reference Cicchetti and Handley2017; Efstathopoulos et al., Reference Efstathopoulos, Andersson, Melas, Yang, Villaescusa, Rȕegg and Lavebratt2018; Murgatroyd, Quinn, Sharp, Pickles, & Hill, Reference Murgatroyd, Quinn, Sharp, Pickles and Hill2015; Oberlander et al., Reference Oberlander, Weinberg, Papsdorf, Grunau, Misri and Devlin2008; Parade et al., Reference Parade, Ridout, Seifer, Armstrong, Marsit, McWilliams and Tyrka2016; Parent et al., Reference Parent, Parade, Laumann, Ridout, Yang, Marsit and Tyrka2017; Radtke et al., Reference Radtke, Ruf, Gunter, Dohrmann, Schauer, Meyer and Elbert2011; Romens, McDonald, Svaren, & Pollak, Reference Romens, McDonald, Svaren and Pollak2015; Tyrka et al., Reference Tyrka, Parade, Eslinger, Marsit, Lesseur, Armstrong and Seifer2015; van der Knaap et al., Reference van der Knaap, Riese, Hudziak, Verbiest, Verhulst, Oldehinkel and van Oort2014). In addition, some studies have demonstrated links between NR3C1 hypermethylation and children's psychopathology (Cicchetti & Handley, Reference Cicchetti and Handley2017; Efstathopoulos et al., Reference Efstathopoulos, Andersson, Melas, Yang, Villaescusa, Rȕegg and Lavebratt2018; Parade et al., Reference Parade, Ridout, Seifer, Armstrong, Marsit, McWilliams and Tyrka2016).
ELS is more common among families touched by psychosocial adversities, such as maternal depression, parental stress, and low socioeconomic status (SES). In these adverse conditions, parenting quality is often compromised, leading to increased risk of major forms of ELS, such as emotional and physical abuse and neglect, as well as dysfunctional relationships between parents and child (Heim, Reference Heim, Gellman and Turner2013). For this reason, social programs that aim to support psychosocially disadvantaged families have been implemented in the last decades (Ertem & Organization, W. H., Reference Ertem2012). Parents as Teachers (PAT), founded in 1984 in the State of Missouri (https://parentsasteachers.org/about/), is a parent-training intervention beginning during the perinatal period, and lasting until the child's third birthday (Schaub, Ramseier, Neuhauser, Burkhardt, & Lanfranchi, Reference Schaub, Ramseier, Neuhauser, Burkhardt and Lanfranchi2019).
The underlying theoretical and empirical background of PAT is largely based on Bronfenbrenner's bioecological model. In this model, reciprocal interactions between an individual and its environment, which includes persons, objects, and symbols, are defined as proximal processes that are regarded as the “primary engines” of development (Bronfenbrenner & Morris, Reference Bronfenbrenner and Morris2007). PAT intervenes in these interactions by improving parenting quality and providing enriched learning opportunities for children (Peterson, Luze, Eshbaugh, Jeon, & Kantz, Reference Peterson, Luze, Eshbaugh, Jeon and Kantz2007; Schaub et al., Reference Schaub, Ramseier, Neuhauser, Burkhardt and Lanfranchi2019). Social programs based on Bronfenbrenner's bioecological model have been demonstrated to be effective in reducing disruptive behaviors in children (Schaub et al., Reference Schaub, Ramseier, Neuhauser, Burkhardt and Lanfranchi2019; Taylor & Biglan, Reference Taylor and Biglan1998; Wyatt Kaminski, Valle, Filene, & Boyle, Reference Wyatt Kaminski, Valle, Filene and Boyle2008) and in normalizing cortisol responses, suggesting an effect of these social interventions on HPA axis regulation (Dozier, Peloso, Lewis, Laurenceau, & Levine, Reference Dozier, Peloso, Lewis, Laurenceau and Levine2008; Fisher & Stoolmiller, Reference Fisher and Stoolmiller2008).
To the best of our knowledge, no study has yet investigated the associations between PAT and NR3C1 methylation. During the perinatal period and the first three years of life, DNAm has been demonstrated to be particularly sensitive to ELS and social experiences, especially those occurring in the context of caregiving (Dunn et al., Reference Dunn, Soare, Zhu, Simpkin, Suderman, Klengel and Relton2019; Szyf, Reference Szyf2019). Therefore, it is plausible to assume that social interventions improving conditions for the child in his/her family environment would have a positive impact on the child's DNAm patterns associated with ELS. In this study, we assessed whether children living in psychosocially at-risk families supported by PAT had lower levels of NR3C1 methylation compared to the control group. Before testing this hypothesis, we also assessed the associations between ELS, NR3C1 methylation, and child behavior problems. As found by the studies mentioned above, we hypothesized that NR3C1 hypermethylation would be associated with higher ELS and behavior problems.
Material and Method
This study is part of ZEPPELIN, a longitudinal and randomized controlled trial aiming to (a) recognize children whose development is at risk of being compromised by psychological stressors, and (b) enhance the developmental opportunities of these children in the long term through PAT (Lanfranchi & Neuhauser, Reference Lanfranchi and Neuhauser2013). In a recent study of ZEPPELIN, PAT was effective in reducing child behavior problems in high-risk families (Schaub et al., Reference Schaub, Ramseier, Neuhauser, Burkhardt and Lanfranchi2019). To answer the questions of the present study, we conducted a cross-sectional investigation using a subsample of PAT0-3 composed of families who agreed to participate in biochemical analyses.
The ZEPPELIN study protocol was approved by the State Ethics Committee of the canton of Zurich (Reference Nr. KEK-ZH 2013-0278).
Recruitment and randomization
Families in psychosocially at-risk situations living in the German-speaking part of Switzerland (more specifically, in Canton Zurich) were identified through an interdisciplinary network of family counseling and health professionals (e.g., parent-counseling offices, pediatricians, midwives, social counseling, psychological and psychiatric services), and recruited before or shortly after the birth of the child. Recruitment took place at three project sites in the Canton of Zurich. Families were included in the study if they presented at least two of the following risk factors: (a) parental risks (low level of education, early parenthood, alcohol or drug abuse, sickness and disabilities), (b) familial risks (single parenthood, partnership conflicts), (c) social risks (lack of social integration, dissocial environment), (d) material risks (unemployment, financial problems, confined living space), and (e) child-related risks (high-risk pregnancy, health issues, prematurity). Exclusion criteria were: (a) no permanent residency permit, (b) severe illness or disability of the child, (c) severe illness or disability of the parent requiring inpatient and long-term psychiatric treatment, and (d) other intensive treatments or child protection procedures. The screening was performed using a self-developed questionnaire (Neuhauser et al., Reference Neuhauser, Ramseier, Schaub, Burkhardt, Templer and Lanfranchi2015).
After prospective participants signed informed written consent, approximately one half of the recruited families were assigned to the intervention group (IG), and the other half to the control group (CG). Families signed the informed written consent before knowing to which group they would be assigned. The assignment was performed using stratified block randomization. Strata were project site, cumulative psychosocial risk factors assessed using the Heidelberger Belastungsskala (HBS) (Sidor, Eickhorst, Stasch, & Cierpka, Reference Sidor, Eickhorst, Stasch and Cierpka2012), marital status (single parent: yes/no), and German-language proficiency (interpreter: yes/no) (Schaub et al., Reference Schaub, Ramseier, Neuhauser, Burkhardt and Lanfranchi2019). An additional consent form was signed by parents who agreed to provide saliva of their child in addition to behavioral data. Approximately 50% of the families agreed to provide saliva samples. Mothers in participating families were easier to reach (e.g., by phone calls, emails) (p < .01), and slightly older (M = 30.4 years, standard deviation [SD] = 6) than mothers in families that did not agree to participate in biological analyses (M = 28.9 years, SD = 5.2). However, the two groups did not differ on other relevant study variables, such as SES or maternal depressive symptoms. The resulting IG and CG subgroups did not differ regarding the strata mentioned above. The flowchart of the participants’ progress through the phases of the study is presented in Figure 1. Families assigned to the CG had access to community services, but were not supported by PAT. Incentives such as birthday greeting cards, small birthday presents, and a monetary benefit of 70 CHF for data collection were offered to both experimental groups.

Figure 1. CONSORT flow diagram modified from Schaub et al. (Reference Schaub, Ramseier, Neuhauser, Burkhardt and Lanfranchi2019). 1Criteria for participation were no longer met after randomization and increasing age of the child. 2Insufficient sequencing coverage.
Participants
The final sample for the present study included N = 132 children (CG; n = 60, IG; n = 72), including seven pairs of twins (CG; n = 3, IG; n = 4). Children were aged 3 years (M = 35.5 months, SD = 1.09), and approximately half were female (n = 72, 54,5%). Families were mostly of European origin (71.2%,), and the remaining were originally from Africa, Asia, and Latin America. Notably, the primary language of 79.5% of the children was non-German. The sociodemographic characteristics of the overall sample and of the individual experimental groups are summarized in Table 1.
Table 1. Demographic and biopsychosocial characteristics of the participating children

Note: N = 132, IG, n = 72; CG, n = 60. Methylation is presented in % of methylated cytosines in the assessed CpGs (NGFI-A binding regions of the NR3C1 exon 1F). IG was coded 1, CG was coded 0.
Abbreviations: ADHD = attention deficit/hyperactivity disorder; CG = control group; CpG = cytosine–guanine dinucleotide; IG = intervention group; M = mean; SES = socioeconomic status; SD = standard deviation
* p < .05.
Parents as Teachers
The aim of PAT, which is to enhance children's development, was pursued using four elements. Firstly, home visits during the first 3 years of life conducted by a qualified parent educator, with a minimum of ten visits per year. In this study, members of the IG received, on average, a total of n = 49.9 (SD = 10.1) home visits before data were collected. During the visit, the parent educator worked with the family on three areas: development-oriented parenting, parent–child interactions, and the well-being of the family. The second element comprised nonmandatory group meetings once a month to allow parents to network with other parents and receive information related to educational practices, Parent×Child interactions, and community services for families. The third element was monitoring of the child's general health and development and the final element was support of parents in their integration in the community and the referral of specific needs to other public institutions.
Data collection
The data collection was conducted at the family home, around the child's third birthday. It consisted of self-report questionnaires, mostly filled out by the mothers, and saliva sampling carried out at the end of the collection session. For mothers that were not proficient in German, questionnaires were available in the mother's first language. Descriptive statistics of the study variables, in the overall sample and based on experimental groups, are reported in Table 1.
Maternal depressive symptom
The Brief Symptom Inventory (BSI-18; Spitzer et al., Reference Spitzer, Hammer, Löwe, Grabe, Barnow, Rose and Franke2011) was used to assess self-reported maternal depressive symptoms over the past week. Although the timeframe of the assessment was short, previous studies have demonstrated that differential maternal mood and behavior may induce rapid changes in NR3C1 methylation during early life in the rat and human offspring, even over a few weeks (Murgatroyd et al., Reference Murgatroyd, Quinn, Sharp, Pickles and Hill2015; Weaver et al., Reference Weaver, Cervoni, Champagne, D'Alessio, Sharma, Seckl and Meaney2004). The BSI-18 was developed as a screening tool for assessing psychological distress (somatization, depression, and anxiety) in clinical and nonclinical populations. The depression subscale includes six items rated on a 5-point Likert-type scale (0 = not at all; 1 = a little bit; 2 = moderately; 3 = quite a bit; 4 = extremely) and assesses thoughts of suicide, feelings of loneliness, melancholy, anhedonia, feeling of hopelessness, and feelings of worthlessness. The internal consistency in the present study was excellent (Cronbach's α = 0.92). The mean score of the six items was calculated to obtain an approximately continuous variable. Scores of the BSI-18 assessed a year earlier were also employed in the present study (Cronbach's α = 0.91).
Parental disagreement
The following two items of the Parental Stress Questionnaire (PSQ; Domsch & Lohaus, Reference Domsch and Lohaus2010) related to parenting attitudes in partnership were used as a proxy of parental stress (termed as “parental disagreement” in this study): “My partner and I totally agree on questions about upbringing” and “My partner and I discuss and decide together about parenting tactics”. The rest of the PSQ items were not assessed in the context of ZEPPELIN. For the two assessed items, the internal consistency in the present study was very good (Cronbach's α = 0.81). Items are rated on a 4-point Likert scale (1 = strongly agree, 2 = agree, 3 = disagree and 4 = strongly disagree) and were answered by both mothers who lived with their partner and mothers living alone with their child (single mothers), mostly of whom were still in contact with the father of the child. The mean score of the two items was calculated to create an approximately continuous variable. The scale for the variable scores was reversed to facilitate the interpretation of the results; high scores are indicative of more risk.
SES
To assess the families’ SES we used the well-established International Socio-Economic Index of Occupational Status (ISEI) (Ganzeboom & Treiman, Reference Ganzeboom and Treiman1996). The ISEI was constructed using data from almost 200.000 men and women in 42 countries. This index is a by-product of the mean educational and income levels for each occupation that is converted into ISEI. The ISEI scale ranges from 0 to 100, with greater scores indicating higher SES. Again, we reversed the variable scores in order to facilitate the interpretation of the results; higher scores correspond to higher risk.
Child behavior problems
Child behavior problems were assessed using the well-established Child Behavior Checklist (CBCL 1½–5; Achenbach & Rescorla, Reference Achenbach and Rescorla2000). Parents were asked to rate the child symptoms “now or within the past 2 months”. We used the five Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV)-oriented subscales which measure both children's internalizing and externalizing symptoms. Internalizing symptoms include affective problems (e.g., underactive, lack of energy), anxiety problems (e.g., not wanting to leave home), and pervasive developmental problems (e.g., disturbed by any change in routine). Externalizing symptoms include attention-deficit/hyperactivity problems (e.g., quickly shifting from one activity to another), and oppositional defiant problems (e.g., being aggressive). The five subscales include, collectively, 45 items that are rated on a 3-point scale (0 = not true, 1 = somewhat or sometimes true, 2 = very true or often true). Row scores were standardized in T-scores (M = 50 ± 10).
Saliva sampling
In this study, we used saliva as a source of DNA. Methylation studies that mainly target the human brain rely on postmortem tissues; this presents clear unpractical implications (Rhein et al., Reference Rhein, Hagemeier, Klintschar, Muschler, Bleich and Frieling2015). Therefore, easily accessible peripheral tissue such as blood, buccal cells, and saliva are widely used as a proxy of the brain for assessing DNA methylation in humans. In particular, the use of saliva carries many advantages, including undemanding and minimally invasive collection, as well as easy handling of samples (Yoshizawa et al., Reference Yoshizawa, Schafer, Schafer, Farrell, Paster and Wong2013). Because methylation is known to be cell- and tissue-specific, the choice of tissues can affect results and their interpretation (Fiori & Turecki, Reference Fiori and Turecki2016; Zhang et al., Reference Zhang, Zhou, Lin, Lowdon, Hong, Nagarajan and Wang2013). However, studies assessing methylation–disease associations have demonstrated that methylation in peripheral tissues can reflect results obtained using brain tissue (Turecki & Meaney, Reference Turecki and Meaney2016). For instance, studies assessing NR3C1 promoter methylation in saliva extensively demonstrate a link between ELS, increased methylation, and child or adult diseases (Cicchetti & Handley, Reference Cicchetti and Handley2017; Efstathopoulos et al., Reference Efstathopoulos, Andersson, Melas, Yang, Villaescusa, Rȕegg and Lavebratt2018; Murgatroyd et al., Reference Murgatroyd, Quinn, Sharp, Pickles and Hill2015; Oberlander et al., Reference Oberlander, Weinberg, Papsdorf, Grunau, Misri and Devlin2008; Parade et al., Reference Parade, Ridout, Seifer, Armstrong, Marsit, McWilliams and Tyrka2016; Tyrka et al., Reference Tyrka, Parade, Eslinger, Marsit, Lesseur, Armstrong and Seifer2015), reflecting results obtained by studies using brain tissues (McGowan et al., Reference McGowan, Sasaki, D'Alessio, Dymov, Labonté, Szyf and Meaney2009; Labonte et al., Reference Labonte, Yerko, Gross, Mechawar, Meaney, Szyf and Turecki2012).
Saliva from children in the present study was collected in Oragene tubes (OG-500, DNA Genotek, Ontario, Canada) using the passive-drool method according to the manufacturer's instructions, and stored at 4°C until subsequent biochemical analyses.
DNA methylation
DNA was isolated using the PrepIT-L2P protocol (OG-500, DNA Genotek, Ontario, Canada). Sodium bisulfite conversion was performed with 500 ng of DNA using the EZ DNA methylation kit (Zymo Research, Irvine, CA, USA). We performed amplification of the NR3C1 target sequence (Figure 2) using the following specific primers: frw 5′-TTG AAG TTT TTT TAG AGG G-3′ and rws 5′-AAT TTC TCC AAT TTC TTT TCT C-3′, which included universal primer sequences CS1/CS2 on the 5′ ends (Fluidigm, San Francisco, CA, USA). Cycling conditions were as follows: 95°C for 3 min, then 40× (98°C for 20 s, 60°C for 15 s, 72°C for 15 s), and final elongation step at 72°C for 45 s. Amplicons were then purified using e-gel size selection (Thermo Fisher Scientific, Waltham, MA, USA). Subsequently, indexing with unique single barcodes (Fluidigm, San Francisco, CA, USA) was performed through a second polymerase chain reaction (PCR) (95°C, 3 min, then 10× (98°C, 20 s; 60°C, 15 s; 72°C, 15 s), and final elongation at 72°C for 45 s). Indexed amplicons were pooled and submitted to a final purification to remove dimers and amplification artifacts. The pooled samples were then diluted to a concentration of 2 nM and sequenced on an Illumina Miseq using the v3 kit (Illumina, San Diego, CA, USA).

Figure 2. Upper part: Schematic diagram of noncoding alternative first exon in the NR3C1 promoter region. Lower part: Sequence of the NR3C1 exon 1F, chr5:142,783,586–142,783,903 located in the 5′ untranslated region of the NR3C1. Dashed boxes delineate NGFI-A binding regions described by McGowan et al. (Reference McGowan, Sasaki, D'Alessio, Dymov, Labonté, Szyf and Meaney2009). These CpG sites are the most widely reported in the literature, in both human and animal studies assessing the effect of ELS on NR3C1 methylation. CpG 1–5 correspond to the CpG sites 30–32 and 37, 38 reported by Palma-Gudiel et al. (Reference Palma-Gudiel, Córdova-Palomera, Leza and Fañanás2015). Underlined sequences correspond to the primer's position.
After the sequencing, the software Trimmomatic v0.35 (licensed under GPL V3 and available at http://www.usadellab.org/cms/index.php?page=trimmomatic) was employed to identify and remove low-quality products (Bolger, Lohse, & Usadel, Reference Bolger, Lohse and Usadel2014). The Bismark program (v0.19.0) was used to extract the counts of methylated (cytosines) and unmethylated (thymine) bases. After summing up methylated and unmethylated counts, we only kept samples showing a coverage (=total counts) of at least 100× (Chen et al., Reference Chen, Gross, Lutz, Vaillancourt, Maussion, Bramoulle and Ernst2017). Three samples did not reach this threshold and were therefore excluded. For the remaining samples, coverage ranged from 2,539× to 125,583×, with a mean of 57,956× ± 29,040. In this study, we analyzed five CpG sites located within the NGFI-A binding regions of exon 1F (Figure 2). The NGFI-A is a powerful regulator of the expression of the GR, and there is consistency for increased methylation of the CpG sites located in the NGFI-A binding regions among association studies focusing on NR3C1 methylation and ELS (Turecki & Meaney, Reference Turecki and Meaney2016). Table 1 presents the average methylation and SD for each of the five CpG sites. One participant showed extremely high percentages of methylation at CpG 5 (86.9%) (M = 0.45%, SD = 2.1). This value was not considered as an outlier, as sufficient coverage was reached at this CpG site (9,531×). Moreover, the other CpG sites for this sample, including the proximal CpG 4, had low methylation (0.13%), which suggests successful completion of the bisulfite reaction. Finally, other studies found high methylation at this CpG, such as 52,17% (Efstathopoulos et al., Reference Efstathopoulos, Andersson, Melas, Yang, Villaescusa, Rȕegg and Lavebratt2018). However, we conducted all statistical analyses with and without the participant showing high percentages of methylation at CpG 5. Results were largely unchanged after the exclusion of this participant and conclusions were not affected.
Statistical analyses
We first performed pairwise Pearson's correlation between all variables: ELS, behavior problems, and NR3C1 methylation (Table 2). Linear regression was used for assessing the predictive effect of ELS and PAT on NR3C1 methylation. Because NR3C1 methylation was strongly positively skewed, we performed log transformation in order to approach normality. Partial correlation was used for assessing the association between NR3C1 methylation and child behavior problems. As NR3C1 methylation was not normally distributed, correlation analyses were performed using a nonparametric (Spearman) correlation. In order to assess whether NR3C1 methylation contributed as a mediator of ELS on child behavior problems, path analysis was conducted using PROCESS macro model 4 (Hayes, Reference Hayes2017). Predictor and outcome variables were included in the path analysis if they were significantly associated with the mediator. Current maternal depressive symptoms were not used in the mediation analysis, as the outcome variables (child behavior problems) were essentially measured before the predictor variable, posing a problematic temporal ordering. Child age at the time of saliva sampling (=child age), child age at the beginning of the intervention (=child age at randomization), sex, and geographical origin were included as covariates in all statistical analyses. The “PAT experimental group” variable was also included as a covariate in all statistical analyses, to control for possible effects of the intervention on the results. All methylation analyses were performed using the mean methylation of the five CpG sites within the NGFI-A binding regions (mean NGFI-A methylation) and methylation levels at individual CpG sites (CpG 1 to CpG 5). The mean composite of CpG sites was used in the statistical analyses, despite the lack of intercorrelation among CpG sites (Table 2). Indeed, the five CpG sites are located in the binding regions of the transcription factor NGFI-A. Therefore, physical NGFI-A binding impedance and consequent alteration in gene expression may result from the average level of methylation at the five CpG sites, rather than from the level of methylation at one single CpG site of the NGFI-A binding region. The percentage of missing values across the 14 variables varied between 0 and 16.7%. In total 165 out of 1,683 data points (8.93%) were incomplete. Questions related to the CBCL questionnaire were not answered by 21 families, while the ELS variables showed nonsystemic patterns of missing data. We used multiple imputation to create and analyze 50 multiply imputed datasets to ensure that a reliable estimate of the fraction of missing information was obtained (Madley-Dowd, Hughes, Tilling, & Heron, Reference Madley-Dowd, Hughes, Tilling and Heron2019; Van Buuren, Reference Van Buuren2018). Incomplete variables were imputed under the missing at random (MAR) assumption, using the default settings of SPSS (Enders, Reference Enders2010). We compared the results using the pooled imputed datasets with the results of complete cases and found no difference regarding the findings. Analyses were conducted using SPSS (IBM statistic, version 24.0, Armonk, NY: IBM Corp.). Mediation was considered significant when the 95% asymmetric confidence interval (CI) generated by bootstrapping did not include the value 0. In regression and correlation analyses, a p value below 0.05 was considered statistically significant.
Table 2. Bivariate correlation between ELS variables, child behavior problems and NR3C1 methylation
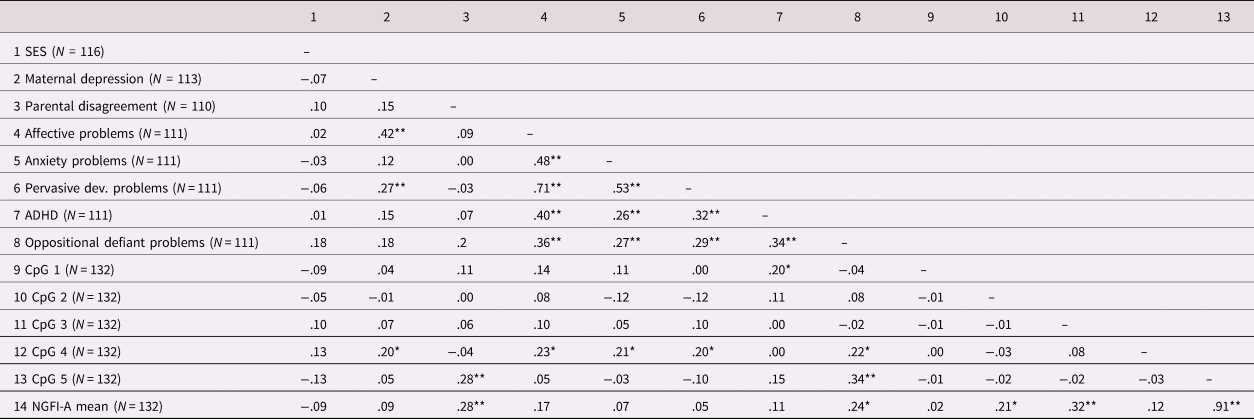
Note: Pearson's correlation.
Abbreviations: ADHD = attention deficit/hyperactivity disorder; CpG = cytosine–guanine dinucleotide; Pervasive dev. problems = pervasive developmental problems; SES = socioeconomic status
* p < .05.
** p < .001.
Results
ELS, PAT, and NR3C1 methylation
Increased mean NGFI-A methylation was predicted by higher current maternal depressive symptoms (β = .19, t(106) = 2.02, p = .046) and higher levels of parental disagreement (β = .30, t(103) = 3.37, p < .01), while it was not predicted by SES (β = .03, t(109) = .28, p = .778), and by maternal depressive symptoms assessed one year before data collection (β = −.02, t(102) = −.23, p = .816). Examination of individual CpG sites revealed significant effects of current maternal depressive symptoms on methylation at CpG 1 (β = .21, t(106) = 2.27, p = .025), CpG 4 (β = .24, t(106) = 2.59, p = .011), and CpG 5 (β = .20, t(105) = 2.20, p = .030). Parental disagreement was predictive of methylation at CpG 5 (β = .30, t(103) = 3.35, p < .01).
Results on the effect of PAT on NR3C1 methylation indicated that the IG had lower mean NGFI-A methylation, although the effect was not statistically significant (β = −.15, t(123) = −1.66, p = .100). The association was weaker after controlling for parental disagreement (β = −.06, t(103) = −.61, p = .544), and for parental disagreement, maternal depressive symptoms, and SES (β = −.02, t(90) = −.19, p = .853). However, the examination of the individual CpG sites revealed that the IG was associated with significantly lower methylation at CpG 1 compared to the CG (β = −.26, t(123) = −2.9, p < .01). This result remained significant after adjusting for parental disagreement (β = −.24, t(103) = −2.47, p = .021), and for parental disagreement, maternal depressive symptoms, and SES (β = −.22, t(90) = −2.16, p = .032).
We then included all predictors in the statistical models. The relative contribution of each predictor to mean NGFI-A methylation and single CpG sites 1–5 is presented in Tables 3 and 4 respectively.
Table 3. Relative contribution of the ELS variables and PAT experimental group to NGFI-A mean methylation
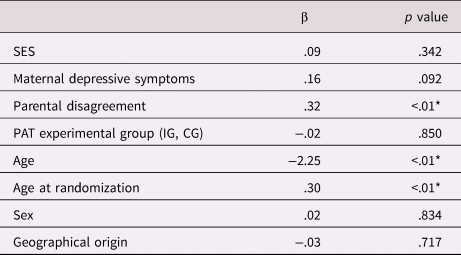
Note: n = 99. IG was coded 1, CG was coded 0.
Abbreviations: CG = control group; IG = intervention group; PAT = Parents as Teachers; SES = socioeconomic status
* p < .01
Table 4. Relative contribution of the ELS variables and PAT experimental group to single CpG sites 1–5 methylation

Note: n = 99. IG was coded 1, CG was coded 0.
Abbreviations: CG = control group; IG = intervention group; PAT = Parents as Teachers; s = CpG sites; SES = socioeconomic status
* p < .05.
** p < .01.
NR3C1 methylation and child behavior problems
Mean NGFI-A methylation was significantly associated with affective problems (rs = .25, p = .012), and oppositional defiant problems (rs = .19, p = .046). Examination of the individual CpG sites revealed significant associations between CpG 5 and affective problems (rs = .24, p = .015), ADHD (rs = .24, p = .014), as well as oppositional defiant problems (rs = .24, p = .013). Table 5 summarizes the results of the associations between behavior problems and NR3C1 methylation.
Table 5. Bivariate correlation between child behavior problems and NGFI-A mean methylation

Note: Spearman's correlation; n = 111; Child age, child age at randomization, sex, and geographical origin and PAT experimental group were controlled in the correlation analyses.
Abbreviations: ADHD = attention deficit/hyperactivity disorder; CpG = cytosine–guanine dinucleotide
* p < .05.
NR3C1 methylation as a mediator
Results indicated that mean NGFI-A methylation significantly mediated the effect of parental disagreement on affective problems (b = .33, SE = .21, 95% CI [.02, .80]), while the direct effect was not statistically significant. The association between parental disagreement on oppositional defiant problems was not mediated by mean NGFI-A methylation, and the direct effect was also not statistically significant. Methylation at individual CpG sites did not mediate the effect of parental disagreement on affective problems or oppositional defiant problems. Table 6 summarizes the results of the mediation analyses between parental disagreement and child behavior problems.
Table 6. Mediation effects of NGFI-A mean methylation between maternal depressive symptoms and child outcomes

Note: n = 98. IG was coded 1, CG was coded 0.
Abbreviations: BootLLCI = bootstrapping lower limit confidence interval; BootULCI = bootstrapping upper limit confidence interval; CG = control group; IG = intervention group; PAT = Parents as Teachers; SE = standard error
* p < .05
Discussion
This study investigated three topics relevant to ELS and NR3C1 methylation in 3-year-old children who lived in psychosocially at-risk families: (a) the effect of ELS (maternal depressive symptoms, parental disagreement, and low SES) on NR3C1 methylation; (b) the associations between NR3C1 methylation and child behavior problems; (c) the question of whether children living in families supported by PAT showed decreased NR3C1 methylation compared to the control group. We found first of all that NR3C1 hypermethylation was significantly predicted by current maternal depressive symptoms (mean NGFI-A methylation, CpG 1, CpG 4, and CpG 5) and parental disagreement (CpG 1 and CpG 5). Secondly we found that NR3C1 hypermethylation was significantly associated with increased affective problems (mean NGFI-A methylation and CpG 5), increased oppositional defiant problems (mean NGFI-A methylation and CpG 5), and increased ADHD (CpG 5). In addition, the association between parental disagreement and depressive symptoms was significantly mediated by mean NGFI-A methylation, Finally we found that children living in families receiving PAT had significantly decreased methylation at CpG 1.
Our findings on NR3C1 methylation associated with ELS are in line with extensive literature reporting on the link between ELS and NR3C1 hypermethylation in animals and humans (Daskalakis & Yehuda, Reference Daskalakis and Yehuda2014; Palma-Gudiel et al., Reference Palma-Gudiel, Córdova-Palomera, Leza and Fañanás2015; Turecki & Meaney, Reference Turecki and Meaney2016; Tyrka, Ridout, & Parade, Reference Tyrka, Ridout and Parade2016). Specifically, increased methylation at CpG 1, CpG 4, and CpG 5 in children has been associated with various forms of ELS, including maternal depressive symptoms (Murgatroyd et al., Reference Murgatroyd, Quinn, Sharp, Pickles and Hill2015; Oberlander et al., Reference Oberlander, Weinberg, Papsdorf, Grunau, Misri and Devlin2008), and parental stress (Radtke et al., Reference Radtke, Ruf, Gunter, Dohrmann, Schauer, Meyer and Elbert2011). Although low SES has been related to ELS (Lefebvre, Fallon, Van Wert, & Filippelli, Reference Lefebvre, Fallon, Van Wert and Filippelli2017), we did not find evidence for an association between NR3C1 methylation and SES. However, this analysis was possibly hampered by the fact that most of the families had low SES (M = 34.44, SD = 23.21).
In this study, we also used data on maternal depressive symptoms collected a year earlier as ELS determinant of NR3C1 methylation. Although the correlation between the first and second assessments was moderate (r = .64), we did not find a significant association between maternal depressive symptoms assessed a year earlier and the child NR3C1 methylation. However, as mentioned above, the association between current maternal depressive symptoms and the child NR3C1 methylation was significant. This may suggest that at 3 years of age, NR3C1 methylation levels are more strongly associated with current or recent maternal symptoms, compared to symptoms assessed a year earlier. These results may also indicate that, between the second and third years of life, NR3C1 methylation is still sensitive to maternal mood, suggesting a window of opportunity for interventions.
Our findings on the association between NR3C1 hypermethylation and adverse child behavior problems, including both internalizing and externalizing symptoms, are consistent with previous research. Increased methylation at CpG 1, CpG 4, and CpG 5 has been associated with various adverse behavioral outcomes in children, including increased externalizing symptoms, higher levels of ego under control, higher levels of emotional lability-negativity (Cicchetti & Handley, Reference Cicchetti and Handley2017), and greater depressive symptoms (Cicchetti & Handley, Reference Cicchetti and Handley2017; Efstathopoulos et al., Reference Efstathopoulos, Andersson, Melas, Yang, Villaescusa, Rȕegg and Lavebratt2018; Parade et al., Reference Parade, Ridout, Seifer, Armstrong, Marsit, McWilliams and Tyrka2016). As reported in the study by Cicchetti and Handley (Reference Cicchetti and Handley2017), we did not find evidence for an association between NR3C1 methylation and anxiety symptoms. Also, we did not find NR3C1 methylation to be significantly associated with pervasive developmental disorders.
NR3C1 methylation has been suggested to mediate the development of stress-related disorders, and there is extensive literature on NR3C1 methylation as a mediator of the negative effects of retrospectively assessed ELS in adults (Palma-Gudiel et al., Reference Palma-Gudiel, Córdova-Palomera, Leza and Fañanás2015; Turecki & Meaney, Reference Turecki and Meaney2016). However, only a few studies have investigated NR3C1 methylation as a mediator between ELS and psychopathology in children (Cicchetti & Handley, Reference Cicchetti and Handley2017; Efstathopoulos et al., Reference Efstathopoulos, Andersson, Melas, Yang, Villaescusa, Rȕegg and Lavebratt2018; Parade et al., Reference Parade, Ridout, Seifer, Armstrong, Marsit, McWilliams and Tyrka2016). We used path analysis to test the indirect effect of ELS on child behavior problems via NR3C1 methylation. The analysis revealed significant mediation of mean NGFI-A methylation in the relation between parental disagreement and affective problems. This finding may be traced to the study by Parade et al. (Reference Parade, Ridout, Seifer, Armstrong, Marsit, McWilliams and Tyrka2016), which demonstrated significant mediation of NR3C1 methylation in the association between ELS (maltreatment) and internalizing behavior problems. As in the present study, externalizing and internalizing symptoms were reported by parents using the CBCL instrument, the sample was ethnically and racially diverse, and included preschool children living in psychosocially at-risk situations.
DNA methylation influences gene expression and is involved in the etiology and phenotypic variation of various physical and mental diseases (Robertson, Reference Robertson2005). One of the most attractive aspects of DNA methylation for translational prevention science is that, unlike the genetic sequence itself, aberrant DNA methylation patterns are potentially preventable and reversible (Szyf & Bick, Reference Szyf and Bick2013). Hence, by modifying at-risk DNA methylation patterns, interventions may reduce the likelihood of disadvantaged phenotypes and promote healthy outcomes (Szyf, Tang, Hill, & Musci, Reference Szyf, Tang, Hill and Musci2016). Our third finding indicates that the IG, receiving PAT, was associated with lower methylation at CpG 1 compared to the CG. This association remained significant after controlling for SES, maternal depressive symptoms, and parental disagreement, suggesting that the mechanism underlying this association was not, or not entirely related to possible effects of the intervention on the ELS variables assessed in this study. The association between PAT and lower NR3C1 methylation in children may be explained by other factors, such as the effect of PAT on parenting quality. Increased methylation at CpG 1 has been related to child and adult psychopathology (Cicchetti & Handley, Reference Cicchetti and Handley2017; Efstathopoulos et al., Reference Efstathopoulos, Andersson, Melas, Yang, Villaescusa, Rȕegg and Lavebratt2018; Parade et al., Reference Parade, Ridout, Seifer, Armstrong, Marsit, McWilliams and Tyrka2016), as well as with suicide in victims who experienced childhood abuse (McGowan et al., Reference McGowan, Sasaki, D'Alessio, Dymov, Labonté, Szyf and Meaney2009). Therefore, our third result suggests that interventions through PAT may play a role in the prevention of mental disorders through an effect on DNA methylation. This result also adds to previous findings suggesting that social interventions impact functional elements of the HPA axis in children exposed to ELS (Dozier et al., Reference Dozier, Peloso, Lewis, Laurenceau and Levine2008; Fisher & Stoolmiller, Reference Fisher and Stoolmiller2008).
Limitations
In this study, we used only two items of the PSQ questionnaire. Hence, only the stress that came with the disagreement on questions about child upbringing was considered. Another limitation may include the lack of NR3C1 methylation data at baseline (pre-intervention) that would have helped to demonstrate the effectiveness of PAT. Finally, we did not assess the NR3C1 BclI polymorphism, which has been associated with increased NR3C1 methylation (Cicchetti & Handley, Reference Cicchetti and Handley2017), and therefore could have influenced the results.
Implications and future directions
DNA methylation markers may provide an objective tool for the early recognition of children exposed and susceptible to ELS, and for monitoring the effectiveness of interventions (Shonkoff et al., Reference Shonkoff, Garner, Siegel, Dobbins, Earls and Wood2012). In addition, a better understanding of how the environment “gets under the skin” may contribute to increased public awareness for the deleterious effects of ELS on health and development, as well as generate support for implementing social interventions, and drive childhood policies and practices (Shonkoff, Reference Shonkoff2016). Our study adds to the literature by showing an effect of the early social environment on NR3C1 methylation measured from saliva of children. More studies are needed in order to confirm the effect of ELS and social interventions on NR3C1 methylation, and identify the core components underlying these associations, such as the frequency, number, and type of ELS exposures causing deleterious effects, as well as critical time windows with respect to the age of the child.
Conclusion
This is the first study to demonstrate an association between a PAT intervention and salivary NR3C1 methylation at a single CpG in children living in psychosocially at-risk situations. This preliminary finding poses avenues for future research interested in the epigenetic effects of social interventions in the context of ELS and the HPA axis. In addition, this is the second study to show that NR3C1 methylation may mediate the association between ELS and internalizing symptoms in preschool children. In this study, we also confirm previous findings on the effect of ELS on increased NR3C1 methylation in children, and on associations between increased NR3C1 methylation and the child behavior problems, including internalizing and externalizing symptoms.
Acknowledgments
The data reported and analyzed in this paper were generated in collaboration with the Genetic Diversity Centre (GDC), ETH Zurich. We would also like to thank Jonathan Lorand for proofreading most parts of this article.
Financial Statement
The findings reported here are based on research conducted as part of the “ZEPPELIN 0–3” research project, funded by the Swiss National Science Foundation [grant numbers 100019_134975, 100019_156610].
Conflicts of Interest
None













