How infant inborn dispositions interact with parental provisions to shape developmental outcomes, the nature versus nurture debate, has preoccupied social scientists for decades. While post World War II perspectives, such as attachment theory, the object relations psychoanalytic school, or even behaviorism, focused mainly on the environment end of the nature–nurture continuum (Bowlby, Reference Bowlby1969; Winnicott, Reference Winnicott1971), later models tended to begin with the dispositional end (Kagan, Snidman, Arcus, & Reznick, Reference Kagan, Snidman, Arcus and Reznick1994), particularly with the dimensions of reactivity and regulation conceptualized as the central domains of infant temperament (Posner & Rothbart, Reference Posner and Rothbart2000). Current neurobiological models attempt to describe specific components in organism and context that chart the field of their mutual effects (Feldman, Reference Feldman2012a, Reference Feldman2012b). For instance, the differential susceptibility model suggests that infant negative emotionality and regulatory abilities define the perimeters of environmental effects, that is, whether parental effects would be extensive or limited (Belsky, Bakermans-Kranenburg, & van IJzendoorn, Reference Belsky, Bakermans-Kranenburg and van IJzendoorn2007). Similarly, epigenetic research on mammalian parenting describes how maternal behavior organizes infant brain oxytocin and glucocorticoid receptor distributions, sculpting both the affiliation and stress systems as well as the capacity to use the former to regulate the latter (Meaney, Reference Meaney2010). These models underscore the organism-context mutual exchange, rather than one pole or the other, as the force that sets the trajectory of optimal versus maladaptive development.
The differential susceptibility and the licking and grooming epigenetic program have each identified specific components in individual and context that are most susceptible to bidirectional effects, thereby charting the field of their mutual influences. From the environment end, the critical component is the species-specific maternal behavior that is adapted online to the infant's cues and changing state. Animal research has shown that well-adapted parenting specifically impacts systems that subserve the management of stress, organization of arousal, and adaptation to the social group (Hofer, Reference Hofer, Golberg, Muir and Kerr1995; Levine, Reference Levine2005; Meaney, Reference Meaney2010) and that functioning of these systems is most sensitive to maternal deprivation in rodents, primates, and humans (Cirulli et al., Reference Cirulli, Francia, Berry, Aloe, Alleva and Suomi2009; Fox, Almas, Degnan, Nelson, & Zeanah, Reference Fox, Almas, Degnan, Nelson and Zeanah2011; Hofer, Reference Hofer, Golberg, Muir and Kerr1995; Shannon et al., Reference Shannon, Schwandt, Champoux, Shoaf, Suomi and Linnoila2005). From the dispositional end, the dimensions of negative reactivity and emotion regulation (ER) have been described as most susceptible to environmental effects (Calkins & Fox, Reference Calkins and Fox2002; Feldman & Eidelman, Reference Feldman and Eidelman2009; Pluess & Belsky, Reference Pluess and Belsky2011). These two components, infant ER and reciprocal parenting, are thus identified as the main players in the field of individual-context mutual influences, the correlational matrix of elements in organism and environment that are susceptible to each other's effects, which stands in contrast to more canalized developmental lines less open to bidirectional impact (e.g., motor development; Gottlieb, Reference Gottlieb1991). Although long-term predictions from parent–child reciprocity and infant ER have been previously studied, few studies have examined their bidirectional dynamics: how infant ER and parental reciprocity interact over time to support long-term outcomes.
Three Mechanisms of Developmental Continuity
There are several mechanisms by which the mutual influences of parenting and infant regulation may underpin long-term outcome. The first considers the individual stability of both ER and reciprocal parenting.Footnote 1 All developmental theories, whether from the social–emotional environment viewpoint or from the temperament perspective, suggest that stable patterns of behavior, modes of “being in the world,” persistent social conducts, or the set of traits that define a personality are formed on the basis of repeated early experiences that are encoded in the brain and engrained in a distinct behavioral style (Bowlby, Reference Bowlby1969; Caspi, Reference Caspi, Damon and Eisenberg1998; Edelman, Reference Edelman1989; Kagan, Reference Kagan, Brim and Kagan1980; Rutter, Reference Rutter1989). Longitudinal studies have shown that ER skills are individually stable across early childhood and predict social adaptation, anxiety, stress reactivity, externalizing and internalizing symptoms, and health outcomes across childhood and adolescence (Calkins & Fox, Reference Calkins and Fox2002; Feldman, Reference Feldman2009; Williams et al., Reference Williams, Degnan, Perez-Edgar, Henderson, Rubin and Pine2009). Sensitive parenting, particularly the dimensions of reciprocity, mutual responsiveness, or synchrony, which define the joint dyadic component of social interactions, are similarly shown as stable over time in research spanning infancy to adolescence (Feldman, Reference Feldman2007a, Reference Feldman2010; Kochanska & Aksan, Reference Kochanska and Aksan2004; Rossi & Rossi, Reference Rossi and Rossi1990; Waters, Merrick, Treboux, Crowell, & Albersheim, Reference Waters, Merrick, Treboux, Crowell and Albersheim2000) and transcend to other relationships in the child's life, such as close friends (Feldman, Bamberger, & Kanat-Maymon, Reference Feldman, Bamberger and Kanat-Maymon2013; Feldman, Gordon, et al., Reference Feldman, Gordon, Influs, Gutbir and Ebstein2013), familiar caregivers (Feldman & Klein, Reference Feldman and Klein2003), or romantic partners (Sroufe, Reference Sroufe2005; Waters et al., Reference Waters, Merrick, Treboux, Crowell and Albersheim2000). Attachment research has underscored the effects of reciprocal parenting on attachment security (Isabella & Belsky, Reference Isabella and Belsky1991) and through such security to a host of positive outcomes, some extending into adulthood (Carlson, Sroufe, & Egeland, Reference Carlson, Sroufe and Egeland2004; Sroufe, Reference Sroufe2005). The long-term effects of both ER and parent–child reciprocity ride on their individual stability; once a trait is stable over time early influences on its functioning will have long-term impact owing to such stability.
The second mechanism relates to the moderating effects of the organism-context exchange on outcome. This mechanism addresses the critical role played by the field of mutual influences between the infant's regulatory abilities and the parent's attuned response in gradually shaping development. Although research has emphasized the moderating effects of contextual factors on continuity in psychopathology over long periods, for example, in shaping trajectories of externalizing behavior from 5 to 27 years (Petersen, Bates, Dodge, Lansford, & Petit, Reference Petersen, Bates, Dodge, Lansford and Petit2014), we know of no study that has focused on bidirectional effects starting at birth and employing repeated observations of both child and parenting. Birth conditions that alter the infant–context interface provide a useful window to this mechanism. For instance, maternal postpartum depression limits the mother's capacity to provide reciprocal parenting (Wienberg & Tronick, Reference Wienberg, Tronick and Noshpitz1997) and the reduction in reciprocity disrupts the emergence of infant ER, leading to higher negative emotionality and ineffective regulation in children of depressed mothers (Blandon, Calkins, Keane, & O'Brien, Reference Blandon, Calkins, Keane and O'Brien2008; Maughan, Cicchetti, Toth, & Rogosch, Reference Maughan, Cicchetti, Toth and Rogosch2007). Similarly, premature birth alters the infant–context exchange because of the infant's immature brain at birth, which results in low physiological regulation (Doussard-Roosevelt, Porges, Scanlon, Alemi, & Scanlon, Reference Doussard-Roosevelt, Porges, Scanlon, Alemi and Scanlon1997), high negative emotionality (Feldman, Reference Feldman2007b), and low regulation (Jean & Stack, Reference Jean and Stack2012), and these in turn disrupt the mother's capacity to read the infant's signals and establish a reciprocal dialogue (Eckerman, Hsu, Molitor, Leung, & Goldstein, Reference Eckerman, Hsu, Molitor, Leung and Goldstein1999; Poehlmann et al., Reference Poehlmann, Schwichtenberg, Bolt, Hane, Burnson and Winters2011). Both conditions, each from a different end, alter the bidirectional field and each has been shown to predict greater psychopathology and compromised social–emotional growth (Apter-Levi, Zagoory-Sharon, & Feldman, Reference Apter-Levi, Zagoory-Sharon and Feldman2013; Essex, Klein, Cho, & Kalin, Reference Essex, Klein, Cho and Kalin2002; Feldman & Eidelman, Reference Feldman and Eidelman2009; Feldman, Rosenthal, & Eidelman, Reference Feldman, Rosenthal and Eidelman2014; Halligan et al., Reference Halligan, Cooper, Fearon, Wheeler, Crosby and Murray2013; Williams et al., Reference Williams, Degnan, Perez-Edgar, Henderson, Rubin and Pine2009). Since the field of mutual influences opens as soon as an infant is born, this mechanism also underscores the need to begin investigations at birth or as close to it as possible and invokes notions of “sensitive periods” (Hensch, 2005) in the formation of optimal fittedness between the organism and its ecological niche (Barrett, Blumstein, Clutton-Brock, & Kappeler, Reference Barrett, Blumstein, Clutton-Brock and Kappeler2013; Feldman, Reference Feldman2015).
The third mechanism differs from the previous two, which describe gradual effects starting with minor birth variations slowly augmented by repeated iterations, by postulating direct unmediated links over lengthy periods. Certain biological states or birth experiences may have imprinting-like effects that directly impact long-term outcomes, often when maturational processes open the possibilities for the expression of these effects. Birth biomarkers potentially included in this mechanism involve general support systems, particularly brain stem mediated systems that underpin multiple regulatory functions along the neuroaxis, or newborn state regulation that shapes the neonate's orientation to the world (Geva & Feldman, Reference Geva and Feldman2008; Nugent, Petrauskas, & Brazelton, Reference Nugent, Petrauskas and Brazelton2009). Overall, these processes have been conceptualized as three mechanisms of developmental continuity and change, including (a) continuity in small steps in which the general stability of a developmental line gradually unfolds over time; (b) continuity via a mediating factor, including the infant–context bidirectional effects as the mediating mechanism; and (c) direct continuity over long periods (Feldman, Reference Feldman2007c). Evidence for each mechanism may be found in longitudinal studies, and they are thought to provide an overall frame for research on continuity, change, and intervention timing (Feldman et al., Reference Feldman, Rosenthal and Eidelman2014).
Dynamic Systems Theory as a Framework to Study the Infant–Context Exchange Over Time
Dynamic systems theory, a perspective rooted in biology and applied to developmental science (Fogel, Reference Fogel1993; Oyama, Reference Oyama1985; Thelen & Smith, Reference Thelen and Smith1994), may provide the most fitting language to describe the bidirectional effects of infant and context over time. The central premise of the theory is that systems self-organize online from organism and context that jointly contribute to system maturation. Thus, the field of mutual influences between the organism's environment-sensitive systems and the contextual elements impacting upon them is the focus of inquiry. Second, dynamic systems models emphasize time as an indispensable parameter of self-organizing systems (Van Geert, Reference Van Geert1994). Hence, these models provide a good framework for assessing the person–context exchange over time. Third, dynamic systems theory provides the most compelling argument for the critical importance of birth conditions for the system's mature profile and demonstrates in multiple areas, from embryology to meteorology, that minor variations in initial conditions can lead to substantial differences over time (the “butterfly effect”; e.g., Smith, Reference Smith2014). Overall, the blurring of distinction between organism and environment, the dependence of the infant on its external-regulatory context, and the conceptualization of attractor states as distinct organism–context combinations that become entrenched over time and constrain the system's future options render this theory the most elegant and parsimonious frame for the study of infant ER and parenting over time. Both parsimony and elegance are important parameters to consider when pitting one theory against another in the explanation of observed data (Popper, 1959/Reference Popper2005).
Heterotypic Continuity in Reciprocity and ER
While both regulatory functions and parent–child reciprocity are individually stable over time, such stability is of the heterotypic type (Caspi, Reference Caspi, Damon and Eisenberg1998; Kagan, Reference Kagan, Brim and Kagan1980; Werner, Reference Werner and Harris1957), implying stability in a hypothesized construct or phenomenological meaning, not in exact overt behaviors.
Reciprocity, an experience learned within early attachments, denotes a social exchange that integrates inputs from multiple partners into a unified social event (Feldman, Bamberger, & Kanat-Maymon, Reference Feldman, Bamberger and Kanat-Maymon2013). Across mammalian species, reciprocity is considered a cornerstone of adaptive social life by providing the basis for social collaboration within groups and enhancing survival through coordinated action (Hauser, McAuliffe, & Blake, Reference Hauser, McAuliffe and Blake2009; Rosenblatt, Reference Rosenblatt and Foss1965). Reciprocity, therefore, is an experience infants must partake of during an early period of neuroplasticity in order to become collaborative members of their social world (Feldman, Reference Feldman2012b). Reciprocal interactions undergo substantial modifications from infancy to adulthood and transit from interactions that require greater amounts of parental reciprocity, the parent's adaptation to the infant's pace and rhythms, to a more balanced give and receive mutuality. In the first months of life, parents adapt to their infant's nonverbal signals in the gaze, affect, vocal, and touch modalities to form the first social exchange. During the second year, parents and toddlers engage in the first verbal–symbolic matched dialogue, and in the preschool years, increasing language skills enable dyads to coconstruct affect-laden narratives. With the emergence of conceptual thought in adolescence, parents and adolescents can discuss conflicts with empathy and perspective taking, and finally, individuals develop reciprocal adult–adult relationships with children, friends, and partners that build on familiarity with the partner's nonverbal patterns and are expressed by mutual self-disclosure, empathy, and care (Feldman, Reference Feldman2007a; Feldman et al., Reference Feldman, Bamberger and Kanat-Maymon2013; Schneiderman, Zagoory-Sharon, Leckman, & Feldman, Reference Schneiderman, Zagoory-Sharon, Leckman and Feldman2012). Phenomenologically, reciprocal exchanges across the lifespan encompass a give and receive quality, construct online from the inputs of both partners, and promote positive affect, security, and intimacy.
Defined as “the extrinsic and intrinsic processes responsible for monitoring, evaluating, and modifying emotional reactions … to accomplish one's goal” (Thompson, Reference Thompson1994), ER is a multifactorial construct, including biological, behavioral, attentional, and cognitive components that hierarchically organize to form response to external or internal events (Calkins & Fox, Reference Calkins and Fox2002). Like reciprocity, ER is expressed by different overt behaviors across maturational stages (Kopp, Reference Kopp1982; Zelazo, Reference Zelazo2004). At birth, ER abilities are supported by physiological systems, particularly periodicities that cycle between states of “on” and “off,” such as heart rhythms or sleep–wake cycles, and by inborn reflexive support of state regulation (Geva & Feldman, Reference Geva and Feldman2008). Emotion regulation across the first year involves the ability to manage negative states and return to baseline following perturbation (Braungart-Rieker & Stifter, Reference Braungart-Rieker and Stifter1996). During the second year, along with the maturation of rudimentary attention and inhibitory control (Diamond, Reference Diamond2002; Gunnar & Davis, Reference Gunnar and Davis2003), children begin to learn social rules, and ER behaviors in socialization contexts describe the capacity to model behavior in accordance with parental requests and prohibitions (Emde, Biringen, Clyman, & Oppenheim, Reference Emde, Biringen, Clyman and Oppenheim1991). By the preschool years, children's ER include self-restraint and the ability to delay gratification (Feldman, Reference Feldman2009; Kochanska, Reference Kochanska1994). Such ER skills support the emergence of regulatory abilities in middle childhood in social and nonsocial contexts expressed through a range of adaptive behaviors, including reduced externalizing and internalizing symptoms, low impulsivity and accident proneness, compliance with social norms, and the capacity to express empathy and resonate with others' distress (Eisenberg et al., Reference Eisenberg, Fabes, Murphy, Maszk, Smith and Karbon1995; Spinrad et al., Reference Spinrad, Eisenberg, Cumberland, Fabes, Valiente and Shepard2006).
The Current Study
The current study addressed the topic of developmental continuity and change, among the central issues in developmental psychopathology (Rutter & Sroufe, Reference Rutter and Sroufe2000), by utilizing a birth cohort observed repeatedly across the first decade of life. The focus was on how infant birth conditions alter the field of infant–context bidirectional effects across early childhood and predict 10-year outcomes. Infant ER and parent–child reciprocity were tested repeatedly in healthy premature infants. As premature infants are prone to regulatory difficulties, it was postulated that such a cohort may shed further light on other birth conditions susceptible to dysregulation.
Regulatory functions at birth were indexed by the two most common indices of regulation in the neonatal stage: autonomic regulation measured by respiratory sinus arrhythmia (RSA) and neurobehavioral regulation measured by the Neonatal Behavior Assessment Scale (NBAS; Brazelton, Reference Brazelton1973). Respiratory sinus arrhythmia, or cardiac vagal tone, measuring the effects of respiration on heart-rate variability as mediated by the parasympathetic system, is a brain stem-controlled pacemaker that reflects the mammalian brain stem organization and provides the foundation for complex behaviors such as attention and social engagement (Porges, Reference Porges2003). Fetal heart rate variability, emerging at 32–34 weeks gestation, is the earliest expression of parasympathetic control and plays a role in the emergence of inhibitory structures (Groome, Loizou, Holland, Smith, & Hoff, Reference Groome, Loizou, Holland, Smith and Hoff1999). Baseline RSA at birth has been shown to predict regulation of negative emotions at 3 months (Huffman, Bryan, & Pedersen, Reference Huffman, Bryan and Pedersen1998), parent–infant synchrony (Feldman & Eidelman, Reference Feldman and Eidelman2007), cognitive development (Doussard-Roosevelt et al., Reference Doussard-Roosevelt, Porges, Scanlon, Alemi and Scanlon1997), attention regulation in the second year (Feldman, Reference Feldman2009), and lower behavior problems at 6 years (Doussard-Roosevelt, McClenny, & Porges, Reference Doussard-Roosevelt, McClenny and Porges2001). State regulation measured by the NBAS indexes the neonate's ability to maintain an organized state and display a range of adaptive states, from sleep to alertness, in the face of incoming visual, auditory, tactile, and multimodal stimuli. Neurodevelopmental regulation differentiates neonates at various levels of risk (Colombo, Moss, & Horowitz, Reference Colombo, Moss and Horowitz1989), predicts infant negative emotionality (Feldman, Reference Feldman2006), visual processing (Moss, Colombo, Mitchell, & Horowitz, Reference Moss, Colombo, Mitchell and Horowitz1988), and cognitive and symbolic development across the first year (Feldman, Eidelman, & Rotenberg, Reference Feldman, Eidelman and Rotenberg2004), and improves following mother–infant skin-to skin contact (Feldman & Eidelman, Reference Feldman and Eidelman2003).
At 3, 6, 12, and 24 months and at 5 years, age-appropriate paradigms were used to measure child ER and mother–child reciprocity, with father–infant reciprocity also observed at 3 months. Such repeated testing enabled the assessment of cross-lagged bidirectional effects as the main area of inquiry. At 10 years, four clusters of outcomes were evaluated, each supported to some extent by regulatory functions: (a) child RSA at 10 years; (b) child accident proneness, which is predicted by low child regulatory capacities in the toddler and preschool years (Schwebel & Plumert, Reference Schwebel and Plumert1999); (c) child empathy as measured by social interaction and a lab paradigm; and (d) externalizing and internalizing symptoms. Empathy, “the capacity to share, understand, and respond with care to the affective states of others” (Decety, Reference Decety2012), develops on the basis of both reciprocal parenting (Feldman, Reference Feldman2007c) and child ER (Wray-Lake & Syvertsen, Reference Wray-Lake and Syversten2011). Zaki (Reference Zaki2014) has recently suggested that individual differences in the motivation to act with empathy depend on regulatory strategies including attention modulation and cognitive appraisal. Externalizing and internalizing symptoms, which have been associated with low regulatory abilities (Eisenberg, Spinrad, & Eggum, Reference Eisenberg, Spinrad and Eggum2010), were reported by mother. An autoregressive cross-lagged structural model was used to examine the continuity of each construct (ER, reciprocity) over time and their cross-time mutual influences as constrained by the system's initial regulatory conditions. Figure 1 presents the overall study model, integrating the conceptual frame and the empirical measures, timing, and hypothesized links.
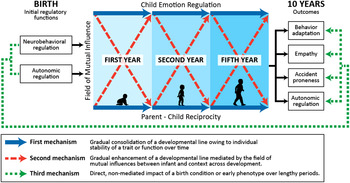
Figure 1. (Color online) Proposed model describing three mechanisms of developmental continuity and charting the long-term effects of birth conditions and the cross-lagged effects of child emotion regulation and parent–child reciprocity on outcomes at 10 years.
The following four hypotheses were proposed. First, both child ER and reciprocal parenting will be individually stable over time. Second, child ER and reciprocal parenting will exert cross-time effects on each other so that higher ER at one stage predicts greater parent–child reciprocity at the next stage and vice versa. Third, each of the four outcomes at 10 years will be predicted by a unique path charted by the mutual influences of ER and reciprocal parenting over time. Such paths will uniquely link more mature regulatory functions at birth with greater empathy, lower behavior problems and accident proneness, and higher RSA via the mediation of the cross-time correlations between child ER and parent–child reciprocity. Fourth, regulatory functions at birth will be directly linked to outcomes at 10 years in addition to the mediated paths via the field of mutual effects across childhood. If substantiated, such a model supports the idea that the infant's regulatory abilities at birth may shape development via both direct long-term impact and the construction of a specific field of individual-context correlations that gradually sculpt development. Notably, and consistent with much prior research (e.g., Choe, Shaw, Brennan, Dishion, & Wilson, Reference Choe, Shaw, Brennan, Dishion and Wilson2014), terms such as bidirectional effects and mutual influences refer only to statistical effects, not to causal effects, because of the correlational nature of the study.
Method
Participants
Children in this longitudinal study were born as healthy premature infants and were followed seven times between birth and 10 years of age. Mothers giving birth to preterm infants (birth weight > 1750 g) in a tertiary-level neonatal intensive care unit who met the study's inclusion criteria were approached to participate. Infants' birth weights ranged from 530 to 1746 g (M = 1448.9 g, SD = 466.02 g) and gestational age was between 25 and 35 weeks (M = 31.46, SD = 3.04). Infants were excluded from the study if they had intraventricular hemorrhage Grades III or IV or suffered from perinatal asphyxia, metabolic, or genetic diseases. All children came from two-parent Israeli Jewish families and all families were considered middle class (Harlap, Davies, Grover, & Prywes, Reference Harlap, Davies, Grover and Prywes1977). At the initiation of the study, mothers were on average 27.96 years old (SD = 5.34) and had completed 14.37 years of education (SD = 2.68). Fathers were on average 30.28 years old (SD = 5.71) with 13.97 years of education (SD = 3.02). Of an initial cohort of 158 premature infants, 125 completed assessment at 10 years and these were included in the current model. Ten children were excluded from follow-up due to sudden infant death (n = 2), diagnosis of autism spectrum disorder (n = 2), and severe motor and/or cognitive delay (n = 6). The remaining 23 children not included in the model did not differ from the participating children on medical, gender, or demographic variables, but they did not have birth data and had minimal data for the first year of life. The final sample included 45% girls. Among children seen at 10 years, 29 were diagnosed with attention-deficit disorder and/or attention-deficit/hyperactivity disorder (23.2%), and of these, 25 were treated by medication. In addition, 9 children (7.2%) had slight motor problems and were treated by occupational/physical therapy. Most children (95.7%) attended typical schools; however, among those in typical schools, 19.4% attended special classes. In terms of behavior problems, 23 children (18.4%) scored above the clinical cutoff on externalizing symptoms and 21 children (16.8%) scored above the cutoff on internalizing symptoms, of which 4 children (3.2%) were above the cutoff on both. The study was approved by the institution review board and all participants signed an informed consent.
Procedure and measures
Children were observed seven times between birth and 10 years: at term age (37 weeks gestational age), at 3, 6, 12, and 24 months (corrected to full gestation), and at 5 years (M = 5.32, SD = 0.60) and 10 years (M = 9.85, SD = 0.86). At each age, multiple tests, experimental paradigms, and parent–child interaction procedures were administered. The current paper utilizes two measures from the neonatal period, RSA and neurobehavioral regulation as measured by the NBAS (Brazelton, Reference Brazelton1973, see below). Children's ER and parent–child reciprocity were coded from age-appropriate paradigms during the next five visits. Four outcomes were measured at 10 years, as described. The neonatal observation took place in the hospital 1–2 days before discharge, the 3-month assessment was conducted in the family's home, and the following assessments were conducted in a developmental laboratory.
Neonatal stage: Physiological and neurodevelopmental regulation
Vagal tone
Autonomic regulation was indexed by vagal tone consistent with Porges’ (Reference Porges2003) model. Approximately 10 min of heart rate were recorded during quiet sleep from the cardiac monitor using a special A/D adaptor that registered the R waves and computed the R-R interval (heart period in milliseconds). The vagal tone index was quantified from the echocardiograph (ECG) output using Porges' MXEdit system (US Pat. 4510944 A, Reference Porges1985) by an assistant trained to reliability at Porges' lab. After editing to remove artifacts, the system converts heart period data into time-based data sampled in 200-ms epochs, determines the periodicities of heart rate with a 21-point moving polynomial, filters the time series to extract the heart period within the frequency band of spontaneous breathing of neonates, and calculates the vagal tone index.
Neurobehavioral regulation
Infants were examined with the NBAS (Brazelton, Reference Brazelton1973) by a trained neonatologist. The NBAS, the most well-validated test for newborns' neurodevelopmental abilities (for a review, see Nugent et al., Reference Nugent, Petrauskas and Brazelton2009), measures the neonate's response to visual, auditory, tactile, proprioceptive, and multisensory stimuli. Items were grouped into six clusters according to Lester's classification (Lester, Reference Lester1984). Two factors were used in the present study to index the infant's inborn regulation, consistent with prior research (Feldman et al., Reference Feldman, Eidelman and Rotenberg2004). The range of state cluster includes four items: peak of excitement, rapidity of buildup, irritability, and lability of state, and the regulation of state cluster includes four items: cuddliness, consolability, self-quieting, and hand to mouth. The range of states and regulation of states clusters were averaged into a single composite (α = 0.74).
ER from 3 months to 5 years
Overall, ER measures were computed as proportions of regulatory indices during age-specific ER paradigms.
First year
3 months
ER was assessed with the Behavior Response Paradigm (BRP; Garcia-Coll et al., Reference Garcia-Coll, Emmons, Vohr, Ward, Brann and Shaul1988). In this procedure, in fants are presented with 17 stimuli in various modalities (e.g., sound, light) and each stimulus is presented for 20 s, with a 10-s break between stimuli. Stimuli are organized in increasing intrusiveness, from simple unimodal (bell sound, flashlight) to aversive, multimodal stimuli (fast-moving car, flashing lights, and loud noise). Infants sat in an infant seat and a trained examiner presented stimuli in a predetermined order. Microanalytic coding was conducted on a computerized system and considered five categories of behavior (gaze, affect, vocalizations, gross and fine motor, and regulatory behavior), each including a set of behaviors. Codes in the regulatory behavior category were based on previous research and included distancing (e.g., arching), gaze aversion from stimulus, autonomic activity (deep or quick breathing and related motor activity; Bazhenova, Plonskaia, & Porges, Reference Bazhenova, Plonskaia and Porges2001), and self-soothing behavior (e.g., hand to mouth, playing with strap of chair) and proportions of these were summed into an ER composite. The use of these age-appropriate regulatory strategies in the face of intrusive or overwhelming stimuli enables infants to maintain a neutral-alert state rather than collapse into a cry or shut-down state and provides an index of regulatory skills. The same ER construct during the BRP has been shown to predict more mature regulatory skills in longitudinal and cross-sectional studies, including exploratory behavior, attention, and information processing, and is improved following skin-to-skin contact (Feldman, Reference Feldman2009; Feldman, Weller, Sirota, & Eidelman, Reference Feldman, Weller, Sirota and Eidelman2002; Harel, Gordon, Geva, & Feldman, Reference Harel, Gordon, Geva and Feldman2011). Reliability was examined for 30 infants and exceeded 88% on all categories. Mean reliability was 93% (κ = 0.81).
6 months
Infant ER was examined during an arm-restraint paradigm. Mothers were asked to interact with the infant for 3 min and then to hold the infant's arm in addition to maintaining a still face for 2 min. Free face-to-face play was then resumed for an additional 2 min. Microcoding was conducted for four categories (gaze, affect, vocalizations, and regulatory behavior). Infant regulatory behaviors were similar to those measured at 3 months with additional behaviors that become available at this age. These included autonomic behaviors, clear gaze aversion from mother's face, distancing (turning around, arching), self-soothing behavior, object manipulation, and diversion tactics (reorienting, nonnegative communications, avoidant behaviors; Braungart-Rieker & Stifter, Reference Braungart-Rieker and Stifter1996) and these were summed to create the ER composite. Reliability was examined for 32 infants and exceeded 86% on all categories. Mean reliability was 92% (κ = 0.79).
12 months
ER was measured during a separation–reunion episode conducted at the end of the visit. Mothers left the room and infants remained with a stranger for 3 min. Mothers then returned and 3 min of mother–child reunion were videotaped. Microcoding was conducted separately for the separation and reunion episodes. The separation codes included four categories: gaze, affect, vocalizations, and activity. Reliability, computed on 28 interactions, exceeded 87% in all categories. Reliability averaged 92% (κ = 0.81, range = 0.70–0.86). Two composites were created for the separation codes on the basis of principal components factor analysis (Feldman & Eidelman, Reference Feldman and Eidelman2004): ER and negative affect. The ER factor loaded on neutral affect (0.89), no vocalizations (0.83), active play (0.75), and toy manipulation (0.71) and these were averaged into the ER construct during maternal separation (α = 0.73).
Second year
The second-year measures address ER abilities in socialization contexts, abilities that develop in the second year of life (Kochanska, Coy, & Murray, Reference Kochanska, Coy and Murray2001). Thus, the child's response to maternal separation measured at 12 months, a regulatory behavior observed already in the first year of life, was included in the first year ER construct, whereas the child's ER observed in a socialization context from the same age was included in the second year construct.
12 months
ER was indexed by the child's self-regulated compliance measured during a toy pick-up paradigm. Following an episode of free play with toys, mothers were given a basket and were asked to have the child pick up the toys. Microcoding considered five regulatory behaviors (self-regulated compliance, externally monitored compliance, noncompliance, defiance, and time-out) consistent with previous research (Feldman & Klein, Reference Feldman and Klein2003; Feldman, Greenbaum, & Yirmiya, Reference Feldman, Greenbaum and Yirmiya1999). Reliability, computed on 25 interactions, exceeded 90% for all categories and averaged 95%, κ = −0.86 (range = 0.80–0.92). Proportions of self-regulated compliance were used to index children's ER, consistent with prior research.
24 months: Toy pick up
ER during toy pick up was measured and coded in accordance with guidelines for the 12-month visit.
24 months: Delayed gratification
We used a party paradigm (Feldman et al., Reference Feldman, Greenbaum and Yirmiya1999) to measure ER. At the end of the visit, mothers and infants were invited to a table set for two with attractive sweets and a bottle of juice. Then, the tester told the child that she forgot the cups, asked mother and child not to touch the sweets until she returned, and left the room for 5 min. Child's ER behavior was measured in a manner similar to the toy pick up paradigm (self-regulated compliance, externally monitored compliance, noncompliance, defiance, and time-out). Reliability was computed for 25 interactions, exceeded 90% in all categories, and averaged 96%, κ = 0.87 (range = 0.81–0.94). Proportions of self-regulated compliance were used.
5 years
Toy pick up
The same procedure and coding used at 12 and 24 months was repeated.
Delayed gratification
Children received an attractively wrapped gift. The tester then left the room for 3 min, put the gift on a table next to the child, and asked the child not to touch the gift until she returned. Microcoding assessed five behaviors: no touch, peek with no reaching, hand reach, touch, and open gift wrap. Reliability was examined for 28 children and exceeded 90% on all categories. Mean reliability was 96% (κ = 0.92). ER included the combined standardized scores of proportions of no touch and latency to the first touch, consistent with prior research (Feldman, Reference Feldman2009).
Standardized scores of the ER measures were averaged into three clusters: ER during the first year included the ER measures from the BRP at 3 months, Still Face at 6 months, and separation–reunion at 12 months. ER during the second year included ER coded from pick up at 12 and 24 months and delayed gratification at 24 months. ER at 5 years included ER coded during the pick-up and delayed gratification tasks at 5 years. In the case of missing data, composites were averaged from the available observations and, in the vast majority of cases (94%), more than one observation was available for each composite.
Parent–child reciprocity from 3 months to 5 years
Overall, reciprocity was measured repeatedly during mother–child interactions. The 3-month visit was conducted in the home and included fathers, thus a measure of father–child reciprocity was available at this age only.
First year
3 months: Mother–child and father–child free play
During a home visit, 5 min of infant–mother and infant–father interactions were separately videotaped in counterbalanced order. Instructions were “play with your child as you normally do.” Mother–child and father–child reciprocity were correlated at a medium level (r = .43, p < .001). Although father–child interactions were available only at one time point, father data were included in order to assess the biparental, stable component of the child's rearing environment.
6 months: Mother–child free play
A 5-min free mother–infant interaction was videotaped during a lab visit. The infant sat on an infant seat, the mother sat next to him/her, and interactions were coded from two cameras using a split-screen generator.
Second Year
12 months: Free play
A 7-min interaction with preselected toys was videotaped. Toys were simple and selected to enhance symbolic play, consistent with our prior research (Feldman, Reference Feldman2007a, Reference Feldman2010). Toys included two dolls; a bottle; a blanket; a tea set including two cups, two plates, sugar bowl and creamer, and a boiler pan; a wallet; a colored necklace; a pair of plastic sunglasses; a sponge; three work tools; two small cars; two telephones; four toy animals, two pet and two wild; and a small tool set.
24 months: Free play
A similar 7-min toy interaction with the same set of toys was videotaped at 24 months.
24 months: Teaching task
Mothers were given a toy that required skills just above the child's developmental level (puzzle) and were asked to help their child play with the puzzle (Dollberg, Feldman, & Keren, Reference Dollberg, Feldman and Keren2010).
5 years
Free play
The same 7-min interaction with toys was videotaped.
Mother–child discussion: “Fun day.”
Mother and child sat across from onr another and were asked to plan a fun day that they could enjoy together (Feldman et al., Reference Feldman, Rosenthal and Eidelman2014).
Coding of all interactions was conducted using the Coding Interactive Behavior manual (CIB; Feldman, Reference Feldman1998). The CIB is a global coding scheme including 42 codes each rated from 1 (low) to 5 (high). The CIB has versions for infants and toddlers, preschoolers, school-aged children, adolescents, and adults, all maintaining, as much as possible, the same codes and constructs. The CIB has good psychometric properties and has been validated internationally in many studies with infants, toddlers, preschoolers, adolescents, and adults in normative and high-risk samples and in longitudinal studies spanning from infancy to adolescence (for a review, see Feldman, Reference Feldman, Mayes and Lewis2012c).
The dyadic reciprocity construct was used here. This construct includes the following codes, reflective of age-appropriate expressions (e.g., mutuality in nonverbal signals in infancy, coconstruction of narrative at 5 years): give and receive reciprocity, fluency, mutual adaptation, and low dyadic tension (α range = 0.85–0.93). At each age, 25 interactions were coded for reliability and reliability averaged intraclass r = .92 (range = .87–.98). As with the ER measures, reciprocity variables were averaged across time: Reciprocity across the first year included mother–infant reciprocity at 3 months, father–infant reciprocity at 3 months, and mother–infant reciprocity at 6 months. Reciprocity across the second year included mother–toddler reciprocity at 12 months during free play, mother–toddler reciprocity at 24 months during free play, and mother–toddler reciprocity at 24 months during the teaching task. Reciprocity at 5 years included mother–child reciprocity at 5 years during free play and joint planning of a fun day. In case of missing data, composites were averaged from the available observations.
Outcomes at 10 years
RSA (vagal tone)
Child RSA was measured from 10 min of baseline ECG. The ECG signals were sampled by a portable ECG monitor-IBI logger system (12 bit, 1000 samples/s/channel, 3992/6 IBI BioLog© System, UFI, Morro Bay, CA). The BioLog system was equipped with active signal-conditioning electrodes attached to participants using three disposable Ag-AgCl skin surface electrode patches. Data were analyzed according to Porges's MXEdit system (US Patent No. 4510944 A, Reference Porges1985), similar to the analysis at birth. The baseline measure was tested prior to a stress paradigm, in which children were exposed to interadult anger.
Child accident proneness
Mothers were interviewed regarding the child's accident proneness and tendency for risky behavior on eight items that were summed into a single score (e.g., frequency of getting hurt at home, falling off bike, etc.; α = 0.71).
Child empathy
This was measured by direct observations and an experimental paradigm. Social neuroscience models of empathy describe empathy as consisting of two subcomponents: “cognitive empathy” and “emotional empathy” (Shamay-Tsoory, Reference Shamay-Tsoory2014), and each was tapped by a different paradigm.
Empathy in dialogue
This was designed to measure the cognitive component of empathy, expressed in the child's ability to understand the mother's side, see her perspective, and display theory of mind abilities toward her views or position. Mother and child were asked to identify a typical conflict in their relationship and discuss it for 10 min. Coding of child empathy considered six codes that evaluated the child's perspective taking and were validated in a longitudinal study (Feldman, Reference Feldman2007c): (a) child sees multiple perspectives: the child shows acknowledgment of mother's (or other persons') thoughts, feelings, desires, wishes, or intentions as differing from those of the self (e.g., “you wanted me to do my homework but I really wanted to play”); (b) child expresses empathy to other's distress: the child shows any form of concern, feelings, or recognition of the mother's sadness, distress, fatigue, or frustration (e.g., “I saw you were tired when you got home”); (c) child is able to change his/her mind in response to mother's comments: the child makes any revision in perspective or action plan throughout the dialogue in reaction to the mother's statements (e.g., “I like to watch the show before doing homework, but next time I'll try to do some homework first”); (d) child accepts criticism: the child shows in any way some form of regret, responsibility, or acceptance when mother points out wrongdoing; (e) child raises a multifaceted solution not based solely on self-interest: the child initiates a solution that considers both own and mother's needs and perspectives; and (f) child uses a dialogical mode of negotiation: the child maintains positive affect and a calm voice, neither withdraws nor intrudes on the mother's speech, and is able to enter into a give and receive exchange that is respectful and shows some consideration. The six scales were averaged into an empathy construct (α = 0.85). The reliability as assessed on 22 sessions was r = .94.
Empathy in response to other's distress
In this paradigm, children observed an emotional vignette from a famous Israeli children's movie (Avia's Summer), which depicts harsh emotional abuse of a new immigrant girl by a group of 10-year-olds living in a youth village in the 1950s. After viewing, children were asked to rate their emotional responses along the following scales, each rated from 1 (low) to 5 (high) degree of empathy toward the victim, level of anger toward perpetrators, sadness felt after seeing the girl's condition, degree of identification with the victim, and desire to console the abused child. Factor analysis identified five items loading on a single factor that included degree of empathy, identification with victim, anger toward bullies, level of sadness to child's distress, and desire to help/console the abused child, and these were averaged into an empathy score. The two empathy scores from the interaction and movie were correlated (r = .41, p < .001), and their standardized scores were averaged into an empathy construct.
Behavior problems
Mother report of the Child Behavior Checklist (CBCL; Achenbach & Edelbrock, Reference Achenbach and Edelbrock1983) was used to measure child behavior problems. The CBCL includes 113 items each rated on a 3-point scale ranging from 0 (never applies) to 2 (almost always applies). Items are clustered into three broad scores: a total behavior score, an internalizing syndrome score, and an externalizing syndrome score. The CBCL is the most widely used instrument for assessing behavior problems in children aged 4–16 years with established reliability and validity. The total CBCL score was used here as the predicted outcome. The total score was used since no hypothesis specifically linked birth regulation with internalizing or externalizing symptoms, but lower regulation at birth was expected to predict greater overall behavior problems.
Results
Prior to model testing, zero-order correlations were computed among all study variables and correlations and descriptive statistics are presented in Table 1. No gender effects were found for any variable and data were collapsed across gender.
Table 1. Intercorrelations, means, and standard deviation of study variables

Note: NBAS, Neonatal Behavior Assessment Scale; ER, emotion regulation; RSA, respiratory sinus arrhythmia; CBCL, Child Behavior Checklist.
*p < .05. **p < .01. ***p < .001.
As seen in Table 1, the neonatal regulatory measures of vagal tone and neurobehavioral regulation were related to infants' ER capacities across the first year, with neonatal vagal tone also associated with parent–child reciprocity across the first year. Both ER and reciprocity were individually stable from 3 months to 5 years and showed some cross time correlations: between reciprocity in the first year and ER in the second year and between ER in the second year and reciprocity at 5 years, and between reciprocity in the second year and ER at 5 years. No direct correlations were found between first year measures and 10-year outcomes. However, neonatal vagal tone was related to higher RSA at 10 years and NBAS correlated with lower accident proneness, higher RSA, and lower empathy at 10 years. Correlations were found between 5- and 10-year measures: ER at 5 years correlated with lower behavior problems and accident proneness, better empathy, and higher RSA at 10 years. Reciprocity at 5 years correlated with greater empathy at 10 years.
An autoregressive cross-lagged model (ARCL; Anderson, Reference Anderson, Arrow, Karlin and Suppes1960; see illustration of model in Figure 1) was computed to examine the main study hypothesis: the effects of neonatal regulatory measures (vagal tone, neurobehavioral regulation) on outcomes at 10 years (RSA, empathy, CBCL, and accident proneness) would be traced to the bidirectional linkage between children's ER and parent–child reciprocity during the first, second, and fifth years. In estimating the ARCL, we controlled for the covariance between children's ER and parent–child reciprocity at ages 1, 2, and 5 years (i.e., time-specific effects), between neonatal regulatory variables, and between outcomes at 10 years. Latent constructs were not used. To estimate the appropriateness of the ARCL, the MPlus 6.1 structural equation modeling package was used (Muthén & Muthén, Reference Muthén and Muthén2010) with maximum likelihood as the estimation method. A model is deemed as having adequate fit to the observed data if the comparative fit index (CFI) and Tucker–Lewis index (TLI) scores are above 0.95, the root mean square error of approximation (RMSEA) is lower than 0.06, and the standardized root mean square residual (SRMR) is lower than 0.07. No data were missing and therefore there was no need to handle missing data as often happens in longitudinal research.
The final model had excellent fit to the observed data, χ2 (28) = 35.24, p = .16, CFI = 0.97, TLI = 0.93, RMSEA = 0.04, RMSEA 95% CI = 0.00, 0.08, SRMR = 0.05, which is presented in Figure 2 (for simplicity, covariates are not presented in the figure). No path was omitted in the process of estimating the model because the proposed model had an excellent fit to the observed data, and no modifications were made to the model (i.e., no covariates were added between variances or residual variances in the model). The fit of the current model was also examined in comparison to a series of alternative models. The model had significantly better fit than an autoregressive model with no cross-lagged paths, χ2 (32) = 58.91, p = .002, CFI = 0.88, TLI = 0.75, RMSEA = 0.08, RMSEA 95% CI = 0.05, 0.12, SRMR = 0.07; Δχ2 (4) = 23.67, p < .0001; an ARCL with equal cross-lagged effects over time, χ2 (30) = 53.98, p = .005, CFI = 0.89, TLI = 0.76, RMSEA = 0.08, RMSEA 95% CI = 0.04, 0.11, SRMR = 0.07; Δχ2 (2) = 18.24, p < .0001; an ARCL with equal autoregressive effects over time, χ2 (30) = 53.16, p = .006, CFI = 0.89, TLI = 0.77, RMSEA = 0.08, RMSEA 95% CI = 0.04, 0.11, SRMR = 0.08; Δχ2 (2) = 17.92, p < .0001; and an ARCL with equal autoregressive and cross-lagged effects per time point, χ2 (34) = 70.37, p = .0002, CFI = 0.83, TLI = 0.68, RMSEA = 0.09, RMSEA 95% CI = 0.06, 0.12, SRMR = 0.09; Δχ2 (6) = 35.13, p < .0001. Coefficients and standard errors of model paths are presented in Table 2. Coefficients and standard errors of model covariates are presented in Table 3. Mediational paths are presented in Table 4.

Figure 2. Results of the autoregressive cross-lagged model assessing paths from infant regulatory capacities at birth to outcomes at 10 years as mediated by the mutual influences of child emotion regulation and reciprocal parenting across development. Model parameter: χ2 (28) = 35.24, p = .16, comparative fit index = 0.97, Tucker–Lewis index = 0.93, root mean square error of approximation = 0.04, root mean square error of approximation 95% confidence interval = (0.00, 0.08), standardized root mean square residual = 0.05.
Table 2. Coefficients and standard errors of model's paths

Note: ER, Emotion regulation; RSA, respiratory sinus arrhythmia; CBCL, Child Behavior Checklist; NBAS, Neonatal Behavior Assessment Scale.
†p < .10. *p < .05. **p < .01. ***p < .001.
Table 3. Coefficients and standard errors of model's covariates

Note: NBAS, Neonatal Behavior Assessment Scale; ER, emotion regulation; RSA, respiratory sinus arrhythmia; CBCL, Child Behavior Checklist.
†p < .10. *p < .05.
Table 4. Total indirect effects via ER and reciprocity

Note: For simplicity considerations we did not report on every mediational path because of the 64 paths linking the neonatal measures with the outcome measures. LB, Lower bound; CI, confidence interval; UB, upper bound; RSA, respiratory sinus arrhythmia; CBCL, Child Behavior Checklist; NBAS, Neonatal Behavior Assessment Scale.
a The mediational path is significant.
The model revealed that the neonatal indices had a direct impact on RSA, empathy, and accident proneness at 10 years. Higher vagal tone and neurobehavioral regulation in the neonatal period were each related to higher RSA at 10 years (NBAS had a marginally significant result). Higher NBAS regulation, but not vagal tone, also predicted lower accident proneness.
As predicted, the neonatal regulatory indices had several significant indirect associations via the bidirectional cross-lagged influences of child ER and reciprocal parenting across the first, second, and fifth years. Bias-corrected bootstrap analyses with 1,000 repetitions indicated that higher vagal tone at birth was related to better RSA (90% CI = 0.01, 0.4), empathy (90% CI = 0.01, 0.11), and accident proneness (90% CI = 0.01, 0.4) at age 10 via improved ER and better parent–child reciprocity. In contrast, NBAS regulation had no indirect links to outcomes at 10 years via ER and parent–child reciprocity across infancy and childhood.
With regard to bidirectional cross-lagged associations between children's ER and parent–child reciprocity at ages 1, 2 and 5 years, the model revealed that, whereas parent–child reciprocity during the first year predicted an increase in ER between the first and second year, ER abilities in the first year did not predict a change in parent–child reciprocity between those time points. On the other hand, between the age of 2 and 5, improved parent–child reciprocity predicted an increase in child ER, and higher child ER capacities predicted an increase in parent–child reciprocity. Overall, the model explained 15.4% of the variance in RSA, 14.8% of the variance in empathy, 9.1% of the variance in CBCL, and 28.4% of the variance in accident proneness.
Discussion
Results of this longitudinal follow-up from birth to 10 years point in several empirical and conceptual directions and have specific implications for early intervention in cases of regulatory difficulties. While numerous studies have followed children across long periods, the current study is unique in three main aspects. First, very few studies following children across the first decade of life have generated such rich and frequent behavioral data that include repeated observations of parent–child interactions and microanalytic assessments of child regulatory behavior. The study therefore advocates the utility of a behavior-based approach to understanding continuity and change. Second, few studies, if any, following children over long time spans have included observational assessments measured at birth. Third, most studies following children over a decade or more have focused primarily on predictors of psychopathology, including externalizing and internalizing symptoms (Diamantopoulou, van Meurs, Verhulst, & van der Ende, Reference Diamantopoulou, van Meurs, Verhulst and van der Ende2009; Petersen et al., Reference Petersen, Bates, Dodge, Lansford and Petit2014) or specific disorders (Frenkel et al., Reference Frenkel, Fox, Pine, Walker, Degnan and Chronis-Tuscano2015; Kagan & Zentner, Reference Kagan and Zentner1996). In contrast, the focus here was on an array of physiological, social, and regulatory outcomes, some more pathology-oriented (CBCL, accident proneness), others indexing social competencies (empathy) or physiological support systems (child RSA).
Overall, the data lend support to the three proposed mechanisms of continuity. In support of the first mechanism, the long-term effects of infant ER and parent–child reciprocity are enhanced by their individual stability. Consistent with the second mechanism, the findings indicate that, in addition to stability, development unfolds as a gradual process in which the infant's regulatory skills and the parent's attuned caregiving sculpt each other over time, creating a dyad-specific field of infant–context attractors that carry long-term effects. It is important to reiterate that the terms effects and influences used here describe statistical, not causal, effects. Finally, in support of the third mechanism, the model reveals direct unmediated paths over the entire first decade, from neonatal RSA and neurobehavioral regulation to outcomes at 10 years. These findings may shed further light on one of the fundamental yet underresearched questions in developmental science: How do infants shape their own growth? The data suggest that infants may influence development via three avenues. First, infants' regulatory abilities at birth trigger the development of regulatory abilities at later stages. Thus, infants who are born with more regulated dispositions will acquire mature forms of regulation with less effort and will move with greater ease from one regulatory task to the next, facing growing environmental challenges with better tools. Second, such infants elicit more reciprocal and well-fitted parental investments that in turn provide essential nutrients for further growth, leading to even finer regulatory outcomes. Third, infants' inborn dispositions may establish a psychobiological birth milieu that can directly impact long-term functioning, possibly through the construction of a more mature and resilient system. These results underscore the importance of studying infant effects on their own growth, a topic that has been neglected in past research. Such research may inform the early detection and treatment for infants prone to dysregulation in order to prevent minor regulatory difficulties from escalating into full-blown regulatory disorders.
Our results indicated that both neonatal measures had direct effects on 10-year outcomes. Neonatal RSA directly predicted child RSA measured at 10 years and NBAS regulation was directly linked to higher empathy, lower accident proneness, and marginally to child RSA. Baseline RSA in the neonatal period has been shown to predict multiple outcomes (Huffman et al., 199; Feldman, Reference Feldman2009; Doussard-Roosevelt et al., Reference Doussard-Roosevelt, McClenny and Porges2001) up to 6 years of age, indicating that the flexibility of the autonomic system, as indexed by cardiac vagal tone at birth, serves as a biomarker of system maturation that taps the underlying substrate that supports regulatory functions (Porges, Reference Porges2003). It is important that RSA was the only measure to demonstrate homotypic rather than heterotypic continuity. The other ER measures were continuous only inasmuch as they addressed the child's skill in handling age-appropriate stressors or socialization demands. The direct links from neonatal state regulation to lower accident proneness and greater empathy is of interest, because we know of no study that has tested 10-year outcomes of NBAS regulation. The neonate's capacity to regulate state in response to the influx of changing environmental stimuli is possibly the neurodevelopmental substrate that supports the child's ability to handle daily obstacles with less impulsivity, greater inhibition, and more planning, thus reducing accident proneness. This neonatal ability also appears to support empathy, perhaps by enabling children to face others' misfortunes with less anxiety, leading to greater motivation to act upon empathic feelings without being overwhelmed.
In addition to direct effects, birth factors predicted development by setting a chain of individual-context processes in a certain direction. Of the two birth measures, only vagal tone showed mediated effects on outcomes and predicted both parent–child reciprocity and infant ER in the first year, which was then reinforced through the stability of these measures over time. Consistent with the vertical-integrative model of regulatory structures of the mind (Tucker, Derryberry, & Luu, Reference Tucker, Derryberry, Luu and Borod2000), these findings highlight the importance of neonatal brain stem mediated functions in setting the stage for both regulatory functions and social competencies across development (Geva & Feldman, Reference Geva and Feldman2008; Porges, Reference Porges2003). Consistent with research using animal models (Hofer, Reference Hofer, Golberg, Muir and Kerr1995), neonatal vagal tone may be a particularly useful index at the prenatal and neonatal stages, describing a critical neurobiological substrate that is open to environmental influences, possibly through the integration of brain stem, limbic, and cortical bottom-up and top-down processes (Levitt, Reference Levitt2003).
Each outcome at 10 years was predicted by a unique set of direct and mediated paths, consistent with the third hypothesis. While RSA at 10 years was directly predicted only by its expression in the neonatal stage (and marginally by NBAS regulation) with no mediated paths, the other three outcomes were predicted by the cross-lagged mutual influences of infant and context, each in a unique way. Child accident proneness, a measure associated with temperamental impulsivity and low inhibition (Schwebel & Plumert, Reference Schwebel and Plumert1999), was predicted by the direct effects of lower vagal tone and NBAS regulation, as well as by the mediated effects of ER at 5 years, that is, how the cumulated effects of ER and reciprocity across early childhood shaped the expression of ER at 5 years. Similarly, children's behavior problems were predicted by ER at 5 years, which in turn was sculpted by the mutual effects of ER and reciprocity across early childhood. It is interesting that empathy was the only outcome predicted by both ER and reciprocity at 5 years, thus influenced by the mutual effects of infant and context on the expression of both child regulation and parent–child reciprocity. Empathy is unique among the current outcomes in that it is a social-regulatory process (Zaki, Reference Zaki2014) that requires regulatory abilities to place others' needs before one's own (Decety, Reference Decety2012; Eisenberg et al., Reference Eisenberg, Spinrad and Eggum2010), but it is also a dialogical ability (Day & Tappan, Reference Day and Tappan1996; Hoffman, Reference Hoffman2000), naturally evolving from reciprocal parenting that places the child's signals at the center of the joint dialogue. Empathy toward peers in adolescence has been linked to parent–child reciprocity across childhood (Feldman et al., Reference Feldman, Bamberger and Kanat-Maymon2013); however, imaging studies have shown that empathy is supported by the dorsolateral prefrontal cortex, which is implicated in inhibition and regulation (Bernhardt & Singer, Reference Bernhardt and Singer2012). Thus, empathy stands out among the outcomes as a developmental skill emerging in early childhood (Thompson, Reference Thompson, Eisenberg and Strayer1987) that draws on the entire field of mutual influences, the way bidirectional effects organize child ER and the way these same factors shape reciprocity of parent and child.
Consistent with dynamic systems theory, the mutual influences of infant and parenting on each other grew tighter over time. This is consistent with the notion that the dynamic integration of organism and context into a unitary system becomes more entrenched and less flexible as systems mature (Thelen & Smith, Reference Thelen and Smith1994). Between the first and second years, the cross-lagged associations were much weaker in magnitude as compared to the correlations between the second and fifth years. Parent–child reciprocity across the first year had a marginal effect on ER in the second year, and infant ER in the first year did not meaningfully predict reciprocity in the second year. However, from age 2 to 5 years, the full bidirectional matrix of ER and parenting was significant. As children grow and the parenting context becomes more fitted to their inner dispositions, particularly to the stable components of such traits, the system's options become increasingly constrained, and parents tend to treat children in a certain way, which in turn elicits a particular regulatory or dysregulatory response from the child. These findings highlight the importance of implementing interventions during the first year of life to enhance both child ER and parent reciprocity before negative cycles of mutual influence become engrained and less open to modifications.
Conceptual implications
Within the global topic of developmental continuity, our study contributes to the discussion on heterotypic continuity, with special implications for social neuroscience. It is important to note that, unless one measures a specific brain structure or physiological system, longitudinal studies of the developing child always involve heterotypic continuity. Even IQ, for which stability is the defining feature, is bound by heuristic continuity rather than consistency in exact behaviors. Constructing a phenomenological line of stable underlying constructs from infancy is among the key contributions of developmental science to the field of psychology: It involves building a theory that narrows down from the multitude of early phenomena those topics, constructs, and behaviors that constitute the trajectory of adaptive functioning in the adult human. Such was the undertaking of the major theories of the 20th century, including those of Freud, Erickson, and Piaget. The importance of longitudinal studies showing predictions from early childhood to adulthood, for instance, from the capacity to delay gratification in preschool to adult employment (Daly, Delaney, Egan, & Baumeister, Reference Daly, Delaney, Egan and Baumeister2015), or from fearful response to novel toys in infancy to behavior inhibition in childhood to anxiety disorders in adulthood (Fox & Pine, Reference Fox and Pine2012), is by naming a childhood phenotype that has meaning for the adult person. What is missing from these models is that the early child phenotype (e.g., fear of novelty) is not a solitary trait but creates ripples in the environment and thus prediction to later functioning should be examined not only from the trait but also from the ripples. Incorporating the field of mutual influences, which charts the dynamic exchange of child dispositions and their impact on parental behavior, punitive approach, or disengagement, may enrich our understanding of heterotypic continuity and improve its predictions. Better definition of age-appropriate phenotypes on key constructs and inclusion of the child–context interface as a mediating factor may reduce error and increase the degree of continuity found in longitudinal research.
The findings also have implications for social neuroscience. To date, studies in social neuroscience have mainly focused on adults and utilized lab-based paradigms. However, several authors have called for the inclusion of paradigms that assess the dynamics of social exchanges in their natural ecology, to move from the study of one-brain activation to two-brain coordination, and to develop a behavior-based approach that can test the brain basis of social participation (Adolphs, Reference Adolphs2010; Singer, 2010). A developmental approach is similarly missing from most social neuroscience research. Studying the maturational process of brain systems that underpin the capacity for social reciprocity as it is expressed across different developmental stages may help describe how the evolution of the social brain enabled homo sapiens to survive and thrive and how disruptions in these networks underpin psychopathologies of social dysfunction, including depression, social anxiety, schizophrenia, or autism (Feldman, Reference Feldman2015).
Several limitations of the study should be considered in the interpretation of the findings. First, the sample size is relatively small for the complex model. While frequent observations and microanalytic coding are difficult to accomplish in very large samples, insights gained from smaller samples should be replicated in larger studies. In addition, as a birth study cannot measure abilities that become available at later stages, our model did not control for initial level of outcomes except in the case of RSA, and this should be noted in the interpretation of the findings. Second, the study was conducted on a population prone to regulatory difficulties. While the developmental psychopathology perspective emphasizes the utility of studying developmental processes in high-risk populations prone to difficulties in the specific domain of investigation (Luthar, Cicchetti, & Becker, Reference Luthar, Cicchetti and Becker2007), examination of the model in full-term infants or other high-risk populations is needed before findings can be generalized. Assessment of other neurobiological systems, including stress and affiliation hormones, brain patterns, or genetic variability, as important birth conditions that constrain systemic development, would have contributed to a fuller picture. Third, inclusion of other outcomes at 10 years could have contributed to a more comprehensive understanding.
Future directions for translating research on the influential child into preventive interventions
The current conceptualization and findings may contribute to translational research and the construction of earlier, more targeted interventions. To further understand the developmental origins of psychopathology, it is important to examine periods of abrupt changes in the child–context interface and test their long-term effects. Such change can stem from natural maturational processes, such as school entry or puberty, which may lead to (potentially temporary) discontinuity in the child's regulatory functions. However, parents may be unable to meet the shift in the child's regulatory abilities and alter their familiar response (e.g., by increasing punishment or surveillance), and this change in the parental approach may further increase child stress or maladjustment. In addition to typical maturational processes, much further research, sophisticated models, and a behavior-based approach is needed to understand how sudden negative events that impact parenting, such as trauma, loss, divorce, or illness, abruptly alter familiar child–parent exchange patterns and how these alterations and their timing in the child's life may compromise growth.
The findings underscore the critical importance of assessing infants' regulatory capacities at birth and beginning treatment as close to birth as possible in cases of risk for regulatory problems. Moreover, the findings specify a unique window of opportunity for interventions that focus on enhancing parent–child reciprocity. Our results indicated that increases in reciprocity during the first year predicted improvement in children's regulatory capacities already at the second year, while the other direction was not significant (change in infant ER did not predict reciprocity in the second year). From the second year onward, the full bidirectional matrix was observed. The first months of life appear to constitute a unique window where every increase in parent–child reciprocity impacts the child's capacity to meet the second year of life, a critical period for cognitive, language, social, and emotional growth, with better tools. Thus, conditions such as premature birth (approximately 10% of births globally) or maternal postpartum depression (~12%–15%) should receive professional help in programs that reach out to parents and focus on promoting regulatory capacities by increasing reciprocity. To date, most parents of premature infants leave neonatal intensive care units with clear instructions as to their infant's motor and cognitive potential delays but have little information about the child's regulatory difficulties or the special form of reciprocity required for infants who send unclear social signals. Finally, as both ER and reciprocal parenting show heterotypic continuity over time, it is important to help parents recognize the specific behavioral expressions of ER at each stage. Parents also need to learn how reciprocal parenting is expressed at each stage and how they can integrate the child's emerging ER abilities into a joint, dyad-specific reciprocal dialogue. Much further research and long-term follow-up is required to understand how infants shape their own development, how parents and infants enter into a dance of mutual influences that becomes more personal and patterned over time, and how birth conditions constrain long-term outcomes at later stages, including the pubertal transition, young adulthood, and the way infants ultimately nurture the next generation.










