Life on earth is governed by the 24-h cycle of light and darkness associated with the rotation of the earth. Normally metabolic and physiological pathways are coordinated to this 24-h cycle by an endogenous clock enabling our bodies to coordinate appropriate physiological processes to the time of day. Research in animals and human subjects suggests that the advent of a 24/7 lifestyle that disrupts our natural sleep cycles and their alignment to the external light–dark cycle is important in the regulation of energy balance and glucose metabolism( Reference Prasai, George and Scott 1 – Reference Tan and Scott 3 ). Sleep curtailment has become a prevalent behaviour in the Western and developing world where it is estimated that average sleep duration has declined by almost 2 h in the past 50 years( Reference Knutson, Van Cauter and Rathouz 4 ). In the USA and UK, a third of the population report getting <7 h sleep per night( 5 , Reference Barnes, Byron and Beninger 6 ). Coinciding with this there has been an explosion in the prevalence of type 2 diabetes mellitus (T2DM), raising the important question of whether there is a causal link between the two. This review will specifically consider the recently published evidence for whether sleep duration is involved in the development of T2DM in human subjects. It will neither appraise the considerable number of studies looking predominantly at the role of sleep in the development of obesity, nor it will address the separate, but related, literature linking sleep-related breathing disorders to obesity and T2DM. Having considered the role of sleep duration per se in the development of diabetes, the evidence for whether sleep duration has a role to play in glucose control in people who have diabetes is reviewed.
Association between sleep duration and the risk of type 2 diabetes
Evidence from prospective studies
Several large cohort studies have investigated the association between sleep duration and the risk of subsequently developing T2DM, studied over varying lengths of follow-up( Reference Yaggi, Araujo and McKinlay 7 – Reference Cespedes, Bhupathiraju and Li 16 ). Details of their study design and outcomes are shown in Table 1. Several big studies in the USA and Germany have shown a U-shaped association between sleep duration and increased risk of T2DM( Reference Yaggi, Araujo and McKinlay 7 – Reference Kowall, Lehnich and Strucksberg 9 ). The studies relied on subjective measures, with sleep duration self-reported at baseline, and T2DM incidence was mainly by self-report of physician's diagnosis. Using 7 h sleep duration per night as a reference category, those with shorter and longer sleep duration were significantly more likely to develop T2DM over the follow-up period of 5–15 years (risk estimates ranging between 1·47 and 1·95 for short sleep duration, and between 1·40 and 3·12 for long sleep duration). Regression models employed in the analysis were adjusted for many potential confounders, mainly; age, physical activity, BMI, alcohol consumption, ethnicity, education, marital status, depression and history of hypertension. Not all studies have shown this U-shaped relationship however. A large Australian study of >192 000 adults used information recorded in medical insurance records( Reference Holliday, Magee and Kritharides 10 ) and reported a positive association between short (but not long) sleep duration and subsequent incidence of T2DM. However, T2DM incidence was determined from hospital admission records and so those who were not admitted to hospital during the follow-up period could not be identified as T2DM, which might have led to underestimation of the actual diabetes incidence. In addition, the follow-up period was relatively short (mean duration 2·3 years). Another prospective study examined the association of sleep duration with development of impaired fasting glucose over 6 years follow-up( Reference Rafalson, Donahue and Stranges 11 ), with 6–8 h sleep duration as a reference category, short (but not long) sleepers had higher odds of developing impaired fasting glucose (OR 3·0, 95 % CI 1·05, 8·59; OR 1·6, 95 % CI 0·45, 5·42 for short and long sleep durations, respectively). A Finnish study in overweight individuals with impaired glucose tolerance found an increased risk of T2DM only in participants with long sleep duration ⩾9 h (hazard ratio (HR) 2·29, 95 % CI 1·38, 3·80)( Reference Tuomilehto, Peltonen and Partinen 14 ). Finally, two recent meta-analyses of nine( Reference Shan, Ma and Xie 17 ) and fourteen( Reference Anothaisintawee, Reutrakul and Van Cauter 18 ) prospective cohort studies have also confirmed the U-shaped relationship (Fig. 1). A couple of other studies have investigated the association between short compared with normal sleep duration and the risk of T2DM without examining for a U-shaped relationship. The first showed that sleeping ⩽7 h per night was associated with higher odds of developing T2DM after 6 years follow-up (OR 1·96, 95 % CI 1·10, 3·50)( Reference Gutierrez-Repiso, Soriguer and Rubio-Martin 12 ). Models were adjusted for age, sex, physical activity, smoking habit, weight gain and abnormal glucose regulation at baseline. The study also found that the odds of becoming obese were significantly higher in subjects who slept ⩽7 h per night (OR 1·99, 95 % CI 1·12, 3·55). There was a lack of association between sleep duration and TD2M at 11 years follow-up that could be related to the attrition in study population over time and to the mediation effect exhibited by adjusting for weight gain in the model. The second study found a higher odds of T2DM after 2 years follow-up, sleeping ⩽5 h compared with >7 h sleep duration (OR 5·37, 95 % CI 1·38, 20·91)( Reference Kita, Yoshioka and Satoh 13 ). The logistic regression models were however adjusted for fasting plasma glucose, an integral feature of the outcome measure, T2DM. This leads to a statistical phenomenon known as mathematical coupling thus rendering the result spurious( Reference Archie 19 , Reference Tu, Maddick and Griffiths 20 ).
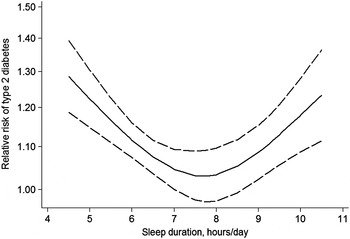
Fig. 1. U-shaped relationship between sleep duration and risk of type 2 diabetes mellitus (adapted from Shan et al.( Reference Shan, Ma and Xie 17 )).
Table 1. Prospective studies on sleep duration and the risk of developing type 2 diabetes mellitus (T2DM)
Extending the understanding of the relationship between sleep duration and risk of T2DM the impact of a change in sleep duration over time has also been investigated. In the Whitehall II study in the UK, change in sleep duration was calculated for participants without diabetes at the beginning and end of four 5-year cycles and T2DM incidence was observed at the end of the subsequent cycle( Reference Ferrie, Kivimaki and Akbaraly 15 ). Another, rather convoluted, prospective study (the Nurses’ Health study) examined whether historic changes in women's sleep duration over the preceding 14 years was associated with developing T2DM over the subsequent 12-year follow-up( Reference Cespedes, Bhupathiraju and Li 16 ). In both studies, logistic regression models showed higher risk of developing T2DM in participants with chronic short sleep duration (⩽5·5–6 h; Whitehall II study OR 1·35, 95 % CI 1·04, 1·76; Nurses’ Health study HR 1·10, 95 % CI 1·001, 1·21) and in those with an increase of ⩾2 h sleep duration (Whitehall II study OR 1·65, 95 % CI 1·15, 2·37; Nurses’ Health study HR 1·15, 95 % CI 1·01, 1·30) compared with those who maintained 7–8 h sleep duration. After adjusting for BMI both associations were attenuated, suggesting that BMI is a mediator in the association. These studies suggest that the adverse metabolic influence of short sleep duration may not be ameliorated by increasing sleep duration later in life.
Taken together, these large prospective studies which include both men and women, and a wide range of ages show that a U-shaped relationship exists between self-reported sleep duration and the development of T2DM. Given the increasing societal pressures to sleep less, these data are very persuasive of short sleep duration being implicated in the co-existent T2DM epidemic.
Evidence from cross-sectional studies
Whilst they do not carry the same weight as prospective studies, there have been several recent cross-sectional studies that suggest there may be some key sociodemographic factors that influence the relationship between sleep duration and T2DM, and these are summarised in Table 2. Again a U-shaped association between sleep duration (over a 24 h period) and T2DM was observed in 130 943 adults, aged 18–85 years, using data from the National Health Interview Survey from 2004 to 2011( Reference Jackson, Redline and Kawachi 21 ). However, short and long sleep durations were more strongly associated with T2DM in white participants than in black. Adjustment for socioeconomic status and other health behavioural factors attenuated the associations in both groups and remained significant only in white participants. An additional cross-sectional study using the National Health Interview Survey data, from years 2004 to 2005, also reported a U-shaped association between sleep duration and T2DM( Reference Buxton and Marcelli 22 ). A Chinese study showed that longer sleep duration over a 24 h period was positively associated with having the metabolic syndrome and T2DM, but only in women( Reference Wu, Xu and Shen 23 ). Objectively measured sleep duration (using wrist actigraphy) showed a significant association between shorter sleep duration and having impaired fasting glucose and diabetes, but did not find any ethnic differences in a multi-ethnic study( Reference Bakker, Weng and Wang 24 ).
Table 2. Cross-sectional studies on sleep duration and the risk of developing type 2 diabetes mellitus (T2DM)

NHIS, National Health Interview Survey; OSA, obstructive sleep apnoea.
Association between sleep duration and the risk of insulin resistance
One of the key features of T2DM is impaired insulin mediated glucose uptake, otherwise known as insulin resistance. This precedes the glucose abnormalities and clinical manifestation of T2DM, often by many years. A selection of both observational and intervention studies have recently explored the relationship between sleep duration and measures of insulin resistance and these are summarised in Table 3.
Table 3. Studies on sleep duration and development of insulin resistance

Evidence from cross-sectional studies
A small study compared the self-reported sleep duration in insulin-resistant individuals (n 35) with that seen in insulin-sensitive individuals (n 21). Those with insulin resistance slept 43 min less per night (P = 0·018). The study also found that 60 % of insulin-resistant participants slept <7 h in comparison with only 24 % only of insulin-sensitive participants (P = 0·013), though no adjustment for potential confounders was performed( Reference Liu, Kushida and Reaven 25 ).
Whilst observational studies are of interest, it is well-designed interventional studies that really help us understand the role of sleep duration in the development of insulin resistance and glucose tolerance. There have been a cluster of these published recently looking at the metabolic effects of both sleep restriction and sleep extension.
Evidence from sleep restriction clinical studies
Multiple small laboratory-based crossover studies on healthy young participants have looked at the effect of sleep restriction on insulin sensitivity and glucose tolerance( Reference Broussard, Ehrmann and Van Cauter 26 – Reference St-Onge, O'Keeffe and Roberts 30 ). Sleep restriction was associated with reduced insulin sensitivity in all the studies (except one( Reference St-Onge, O'Keeffe and Roberts 30 )), with reduced glucose tolerance in one of them( Reference Nedeltcheva, Kessler and Imperial 27 ). The study that found no effect on glucose or insulin aimed to determine the hormonal effects of restricting sleep duration under controlled feeding conditions( Reference St-Onge, O'Keeffe and Roberts 30 ). A controlled diet was provided and the participants lost weight in both the habitual and short sleep phases. It is possible that in the context of negative energy balance, acute short sleep duration does not lead to a state of increased insulin resistance. These studies were all performed in controlled laboratory-based environments and explored acute and often severe sleep restriction. One recent study has examined participants in their home environment to determine if milder and more chronic sleep restriction, akin to daily life, has a role to play( Reference Robertson, Russell-Jones and Umpleby 31 ). Nineteen healthy, young, normal-weight men with habitual sleep durations of 7·0–7·5 h and no sleep disturbances were randomised to either study arm (1·5 h reduction in habitual bedtime) or control arm (habitual bedtime) for 3 weeks. Sleep restriction led to a decrease in insulin sensitivity at the end of first week but then recovered to baseline levels.
In summary, these intervention studies are showing that sleep restriction is associated with the development of insulin resistance and impairment of glucose tolerance. Whether these effects are short-lived adaptive responses to an acute stress, or whether they persist longer term and contribute to the risk of T2DM remains unclear.
Evidence from sleep extension clinical studies
Given that short sleep duration and sleep restriction are linked to the development of insulin resistance, it is timely that a couple of studies are starting to address whether sleep extension has beneficial effects on insulin and glucose metabolism.
The first, a crossover study( Reference Broussard, Wroblewski and Kilkus 32 ) showed that whilst insulin sensitivity deteriorates after acute sleep restriction it recovers after 2 d catch-up sleep. Under laboratory-controlled conditions participants had up to 8·5 h sleep per night for four consecutive nights and up to 4·5 h sleep for another four consecutive nights in a randomised order. After the nights of restricted sleep, participants had two catch-up nights of 10–12 h sleep. Participants had a 23 % decrease in insulin sensitivity after 4 d sleep curtailment compared with normal sleep. However, insulin sensitivity was restored after 2 d catch-up sleep. Although the study showed that catch-up sleep may reverse the negative impact of short-term sleep deprivation, the long-term impact of a repeated sleep deprivation and catch-up sleep cycles on diabetes risk is not known.
The second study investigated whether sleep extension in the home environment has a positive impact on glucose metabolism in healthy adults with chronic sleep curtailment( Reference Leproult, Deliens and Gilson 33 ). Sixteen young healthy non-obese adults, mostly females, had 2 weeks habitual time in bed followed by 6 weeks 1 h/d extension time in bed. Glucose and insulin were assayed at the end of the two periods. During the intervention phase participants mostly went to bed an hour earlier and had higher sleep duration during weekdays but maintained the same sleep duration during weekends. The study indicated no significant difference between pre- and post-intervention fasting glucose and insulin levels, though no statistics were shown( Reference Jones and Kenward 34 ). A moderate linear relationship was reported between the relative change in sleep duration and the relative change in fasting glucose (r = +0·65, P = 0·017) and insulin levels (r = −0·57, P = 0·053); however, we cannot quantify these relationships nor state if they were statistically significant, without a reported estimate measure of associations.
In conclusion, these sleep extension studies, offer tantalising data supporting a potential benefit of sleep extension on glucose control and insulin sensitivity. Much more work is clearly needed in this area.
Association between sleep duration and glycaemic control in patients with diabetes
Given the mounting evidence supporting a relationship between sleep duration and the development of insulin resistance and T2DM, it is relevant to consider whether sleep duration has an impact on glycaemic control in people with established diabetes. Most studies to date are cross-sectional with sample size ranging from as low as eighteen participants to as high as 8543 participants, and these are summarised in Table 4. Most of these studies assessed glycaemic control using HbA1c except one that used capillary glucose levels( Reference Barone, Wey and Schorr 35 ). Sleep parameters were mainly self-reported except for three studies that measured sleep objectively using wrist actigraphy( Reference Barone, Wey and Schorr 35 – Reference Borel, Pepin and Nasse 37 ).
Table 4. Studies on sleep and glycaemic control in patients with diabetes
FPG, fasting plasma glucose; PPG, postprandial glucose; PSQI, Pittsburgh Sleep Quality Index; T2DM, type 2 diabetes mellitus.
Evidence from cross-sectional studies using subjectively reported sleep duration
Okhuma et al. showed that shorter and longer sleep durations were associated with a higher HbA1c level compared with a sleep duration of 6·5–7·4 h in T2DM patients (Fig. 2)( Reference Ohkuma, Fujii and Iwase 38 ). Sleep duration including naps was self-reported. This U-shaped association was not attenuated after adjusting for BMI, total energy intake and depressive symptoms. A similar U-shaped relation was reported in a large Korean study, which included participants with both T1DM and T2DM( Reference Kim, Kim and An 39 ). The association between short sleep duration and poor glycaemic control was strongest for females and participants below the age of 65 years. However, these associations was attenuated after adjusting for BMI and waist circumference and no association was observed after further adjustment for treatment status, duration of diabetes, and daily energy intake. Conversely, only longer sleep duration was associated with poor glycaemic control in T2DM patients in a large Chinese( Reference Zheng, Wang and Pan 40 ) and a smaller Taiwanese study( Reference Tsai, Kann and Tung 41 ). Perceived sleep debt but not sleep duration was positively associated with HbA1c in African Americans with T2DM without diabetes complications and not using insulin( Reference Knutson, Ryden and Mander 42 ).
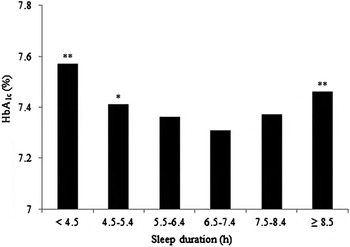
Fig. 2. Higher HbA1c observed in shorter and longer sleep duration in Japanese type 2 diabetes mellitus patients compared with 6·5–7·4 h sleep duration (*P < 0·05; **P < 0·01), adapted from Okhuma et al.( Reference Ohkuma, Fujii and Iwase 38 ).
Evidence from cross-sectional studies using objectively measured sleep duration
Using wrist actigraphy to objectively measure sleep parameters for three consecutive days in the home environment, Trento et al. found no difference in sleep duration between T2DM and control subjects( Reference Trento, Broglio and Riganti 36 ). T2DM sleep quality was found to correlate slightly with glycaemic control, while no correlation with sleep duration was reported. Conversely, Borel et al. shown that T1DM patients with shorter sleep duration (<6·5 h) had a higher HbA1c than those with longer sleep duration (>6·5 h; mean 8·5 v. mean 7·7 %; P = 0·001)( Reference Borel, Pepin and Nasse 37 ). In an adjusted regression model shorter sleep duration was associated with 0·64 % increase in mean HbA1c level compared with longer sleep duration but no 95 % CI or P-value were reported. Participants with short sleep duration were also more likely to have obstructive sleep apnoea and as this was not considered in the analysis, part of the difference reported could be attributed to it.
Finally Barone et al. assessed the association between objectively measured sleep parameters using wrist actigraphy and glycaemic control using capillary glucose levels from glucometer in a group of eighteen young adults with T1DM( Reference Barone, Wey and Schorr 35 ). They showed a positive correlation between the average amount of rest at night and both average glucose levels (r = 0·5404; P = 0·0697) and glycaemic variability (r = 0·5706; P = 0·0527). No estimate measure of the association nor adjustment for any potential confounders were made. Moreover, the average night rest duration was calculated from 10 d actigraphy, which may have included two weekends for some participants and only one weekend for others, and thus it will be inevitably incomparable between participants.
In summary, in people with diabetes there also appears to be evidence of an association between both short and long sleep durations and worse glucose control. However, these studies are cross-sectional and so evidence of causality cannot be inferred. A complicating factor when interpreting the relationship between glucose and sleep in people with diabetes is that extremely poor glucose control is well recognised to cause polyuria, polydypsia and nocturia meaning they are awake during the night. Reverse causality cannot be excluded in these studies as they do not distinguish the severity of glucose control and are likely to include participants experiencing some of these osmotic symptoms. Randomised clinical trials with exposure to sleep duration modification (restriction or extending) and robust methods of assessing temporal glucose across the 24 h day and night( Reference Law, Secher and Temple 43 ) are needed to yield more definitive answers.
Conclusions
The recently published literature of both observational and interventional studies strongly supports a role for both short and long sleep durations in the development of insulin resistance and T2DM. There are insufficient sleep intervention studies to determine whether this risk is modifiable long term. In people with established diabetes the published literature supports an association between poor glucose control and both short and long sleep durations. However, there are no studies that determine the causal direction of this relationship in people with diabetes, nor whether sleep interventions may offer benefit in achieving glucose control. This is a fertile field for future research.
Financial support
A. A. receives financial support from the Ministry of Health, Saudi Arabia.
Conflicts of interest
None.
Authorship
E. S. gave the talk at the RSM and conceived the article. A. A. and E. S. drafted the original document. All authors reviewed and commented on the draft prior to developing the final document.











