Abstract
Introduction
Rheumatoid arthritis (RA), psoriatic arthritis (PsA), and axial spondyloarthritis (axSpA) are three common inflammatory rheumatic diseases that can lead to deformities and joint destruction. Few studies have compared disease burden across patients with these diseases. The objective of this study was to compare disease burden in patients with RA, PsA, or axSpA in routine US clinical practice.
Methods
This study included adults with RA, PsA, or axSpA enrolled in the Corrona RA and PsA/SpA registries between March 2013 and March 2018. Patient and clinical characteristics at enrollment were compared between patients with RA vs. PsA and RA vs. axSpA using t tests or Wilcoxon rank-sum tests for continuous variables and χ2 or Fisher’s exact tests for categorical variables.
Results
A total of 11,350 patients with RA, 2003 with PsA, and 495 with axSpA were included. Patients with RA had shorter mean symptom and disease duration (9.4 and 7.6 years, respectively) than those with PsA (11.2 and 8.4 years) or axSpA (16.7 and 9.8 years). Patients with PsA had lower mean physician global assessment (18.6 vs. 27.3), higher patient global assessment (43.2 vs. 36.9), comparable pain (38.9 vs. 39.5), and lower fatigue (41.1 vs. 43.4) scores than those with RA. Patients with axSpA had comparable mean physician global assessment (25.5 vs. 27.3) and higher patient global assessment (50.2 vs. 36.9), pain (46.1 vs. 39.5), and fatigue (48.3 vs. 43.4) scores than those with RA.
Conclusions
Disease burden in patients with PsA or axSpA was comparable to or greater than that in patients with RA on the basis of common patient-reported outcome measures but appeared lower when assessed using RA disease activity measures, suggesting that disease-specific approaches to care are needed to optimize disease management.
Funding
This study was sponsored by Corrona, LLC, and financial support was provided by Novartis. The Rapid Service Fee was funded by Novartis.
Plain Language Summary
Plain language summary available for this article.
Similar content being viewed by others
Plain Language Summary
Rheumatoid arthritis (RA), psoriatic arthritis (PsA), and axial spondyloarthritis (axSpA) are common rheumatic diseases that can lead to deformities and joint destruction. RA, PsA, and axSpA share many symptoms, including pain, stiffness, fatigue, and reduced physical function, which can lead to a substantial physical and emotional burden on patients with these diseases. Comprehensive assessment of disease burden from both physician and patient perspectives is important for helping make decisions about treatment and disease management. Currently, more research has been done to evaluate the impact of disease on patients with RA compared with patients with PsA or axSpA, and few studies have compared disease burden across patients with these diseases.
This study compared disease burden in patients diagnosed with RA, PsA, or axSpA enrolled in the US-based Corrona RA and PsA/SpA registries. In this real-world population, patients with PsA or axSpA had a longer time from symptom onset to diagnosis than patients with RA, suggesting that PsA and axSpA may not be as well recognized in clinical practice compared with RA. Patients with PsA or axSpA had a disease burden comparable to or greater than that in patients with RA when assessed using common patient-reported outcome measures; however, disease burden in patients with PsA or axSpA appeared lower when assessed using RA disease activity measures. These results provide physicians with important insights into the impact of RA, PsA, and axSpA and highlight the need for disease-specific clinical measures and management strategies to better control disease in patients with PsA or axSpA.
Introduction
Rheumatoid arthritis (RA), psoriatic arthritis (PsA), and axial spondyloarthritis (axSpA) are three common inflammatory rheumatic diseases that can lead to deformities and joint destruction. Despite many overlapping characteristics, these diseases are differentiated by key signs and symptoms, pathogenic mechanisms, and the primary populations they affect.
The estimated prevalence of RA in the United States is 0.5% [1]; prevalence is approximately three times greater in women than men and increases with age [2]. RA is characterized by joint inflammation, tenderness and swelling, and progressive degradation of joint architecture [2]. Joint involvement is often symmetric and commonly affects the small joints in the hands, wrists, and feet [3]. Additional diagnostic indicators for RA include seropositivity for rheumatoid factor or anti-citrullinated protein antibody and elevated erythrocyte sedimentation rates or C-reactive protein levels [4].
Psoriatic arthritis is an immune-mediated, inflammatory arthritis frequently associated with psoriasis. Psoriasis affects approximately 7.4 million adults (2–4%) in the United States [5]; nearly one-third of patients with psoriasis develop PsA, with onset of PsA typically occurring 8–10 years after the onset of psoriasis [6,7,8]. Both psoriasis and PsA affect men and women at approximately equal rates [7, 8]. PsA affects the musculoskeletal system, skin, and/or nails; the symptoms of PsA are diverse and include axial skeleton disorders, nail and skin changes, peripheral joint inflammation, enthesitis, and/or dactylitis [9]. PsA symptoms may occur alone or in combination and range from mild to very severe; this heterogeneity of symptoms and symptom severity can complicate disease management [10]. Additionally, the overlap of PsA symptoms with symptoms of other inflammatory rheumatic diseases may lead to misdiagnosis [11]. Unlike RA, the arthritis associated with PsA is generally asymmetrical, and most patients with PsA are seronegative for rheumatoid factor [8].
Axial spondyloarthritis is the most common form of spondyloarthritis and encompasses both radiographic disease, in which structural damage to the sacroiliac joints is visible on X-ray scans (also known as ankylosing spondylitis), and nonradiographic disease, in which there is no structural damage in the sacroiliac joints [12, 13]. The estimated prevalence of axSpA in the United States is 0.9–1.4%, and onset typically occurs before patients are 45 years of age [12, 14]. However, axSpA is underdiagnosed, and diagnosis is often delayed [15,16,17]; thus, the true prevalence of this disease may be higher than current estimates. Historically, axSpA was thought to be a disease that predominantly affected men, but recent evidence suggests that the prevalence of axSpA may be comparable between men and women [18]. AxSpA primarily affects the axial skeleton, causing inflammation of the spinal vertebrae that can lead to fusion of the vertebral joints, and frequently affects the peripheral joints and entheses [14]. Chronic back pain is the leading symptom of axSpA; other signs and symptoms include arthritis, enthesitis, dactylitis, and extra-articular manifestations such as uveitis, psoriasis, and inflammatory bowel disease [19, 20]. Additionally, test results for HLA-B27 are positive in a high proportion of patients with axSpA [12, 14, 19].
Tumor necrosis factor inhibitors (TNFis) have traditionally been the first choice of biologic therapy for patients with active RA, PsA, or axSpA and have shown efficacy in the improvement of disease activity in patients with active disease despite conventional treatment [10, 20,21,22]. TNF broadly mediates the inflammatory response in RA, PsA, and axSpA; therefore, its inhibition represents a broader approach for treatment of these diseases [2, 14, 23]. However, recent research focusing on the development of novel therapies has deepened our understanding of the unique pathogenic mechanisms of these diseases and identified roles of additional cytokines in disease pathogenesis, including interleukin (IL)-12, IL-17, and IL-23 in PsA and axSpA and IL-6 in RA [2, 14, 23]. The identification of these cytokines as specific key inflammatory mediators has led to the development and approval of targeted therapies that may allow more tailored management of these diseases [10, 20,21,22]. As the therapeutic landscape continues to expand, a thorough understanding of disease pathogenesis, hallmark signs and symptoms, and the patient populations affected by these diseases is essential for accurate diagnosis and effective disease management.
In addition to clinical signs and symptoms, RA, PsA, and axSpA are associated with pain, stiffness, fatigue, and impaired physical function that can decrease health-related quality of life [24,25,26,27]. Patients with RA, PsA, or axSpA also have an increased risk of developing other comorbidities, including cardiovascular disease, infection, cancer, and psychiatric disorders such as depression and anxiety [28,29,30,31,32]. The discomfort and disability resulting from RA, PsA, and axSpA and their associated comorbidities can impose a substantial physical, emotional, and economic burden on patients with these diseases [25, 26, 33].
Comprehensive assessment of disease burden from both clinical and patient perspectives is important for guiding decisions regarding treatment and disease management in patients with RA, PsA, or axSpA. Currently, more research has been conducted to characterize symptom presentation and the impact of disease burden on patients with RA compared with patients with PsA or axSpA. However, few studies have compared disease burden across patients with RA, PsA, or axSpA, in part because there are limited disease measures that are directly comparable across all three diseases. The objective of this study was to compare the disease burden in patients diagnosed with RA, PsA, or axSpA enrolled in the US-based Corrona RA and PsA/SpA registries.
Methods
Study Setting
The Corrona RA registry was initiated in 2001 and has been previously described in detail [34, 35]. Briefly, the Corrona RA registry is a large, independent, prospective, observational cohort that collects longitudinal real-world data from patients and their treating rheumatologists. The registry includes patients recruited by 686 participating rheumatologists from 174 private and academic practice sites across 41 states in the United States. As of March 31, 2018, data on 48,535 patients with RA had been collected. Corrona’s RA database currently includes information from 367,457 patient visits and 169,968 patient-years of follow-up observation, with a mean duration of patient follow-up of 4.30 years (median, 3.35 years).
The Corrona PsA/SpA registry, like the RA registry, is a large, independent, prospective, observational cohort; it was initiated in March 2013 and comprises patients diagnosed with PsA or SpA by a rheumatologist. The registry includes patients recruited by 45 participating rheumatologists from 35 private and academic practice sites across 25 states in the United States. As of March 2018, data on approximately 2827 patients with PsA/SpA had been collected. The Corrona PsA/SpA registry includes information on 11,525 patient visits and approximately 6278 patient-years of follow-up, with a mean duration of follow-up of 3.1 years (median, 3.5 years).
All participating investigators were required to obtain full board approval for conducting noninterventional research with a limited data set involving human participants. The Corrona RA and PsA/SpA registries and their investigators have been reviewed and approved by a central institutional review board (IRB; New England Independent Review Board No. 120160610 [RA] and No. 120160070 [PsA/SpA]). For academic investigative sites that did not receive a waiver to use the central IRB, full board approval was obtained from the respective governing IRBs. All research was conducted in compliance with the Declaration of Helsinki of 1964 and all later amendments. All registry participants were required to provide written informed consent and authorization prior to participating.
Study Population and Data Collection
This study included all patients aged ≥ 18 years with RA, PsA, or axSpA enrolled in the Corrona RA and PsA/SpA registries between March 2013 and March 2018. Data were collected at registry enrollment using questionnaires from patients and their treating rheumatologists and were presented by disease group based on rheumatologist-reported diagnoses at enrollment (RA, PsA, and axSpA; the PsA and axSpA groups were not mutually exclusive). Data collected at enrollment included demographics (age, sex, race, body mass index [BMI], education, insurance type, and work status), clinical characteristics (symptom duration, disease duration, history of comorbidities, and prior medication use), disease activity measures (28 tender joint count [TJC28] and swollen joint count [SJC28], Clinical Disease Activity Index [CDAI], modified Disease Activity Score in 28 joints [DAS28], and physician global assessment), laboratory measurements (C-reactive protein and erythrocyte sedimentation rate), and patient-reported outcome (PRO) measures (pain [visual analog scale (VAS), 0–100], fatigue [VAS, 0–100], morning stiffness, Health Assessment Questionnaire [HAQ; 0–3], and modified HAQ [0–3]).
Statistical Analyses
Statistical analyses were conducted using Stata version 15.0 (StataCorp, College Station, TX, USA). Categorical variables were summarized using frequency counts and percentages; continuous variables were summarized using means and standard deviations. Patient demographics, clinical characteristics, disease activity measures, and PRO scores at enrollment were compared between patients with RA vs. PsA and patients with RA vs. axSpA using χ2 or Fisher’s exact tests for categorical variables and t tests or Wilcoxon rank-sum tests for continuous variables. As a sensitivity analysis, physician global assessment, patient global assessment, patient pain, and patient-reported fatigue were compared between patients with RA vs. PsA and those with RA vs. axSpA using general linear regression models adjusted for symptom and disease duration.
Results
Patient Demographics
A total of 11,350 patients with RA, 2003 patients with PsA, and 495 patients with axSpA were enrolled in the Corrona RA and PsA/SpA registries between March 2013 and March 2018 and were included in the analyses. A total of 49 patients had both PsA and axSpA as reported by their rheumatologists at enrollment.
Patient demographics are described in Table 1. As expected, patients with RA were older than those with PsA or axSpA (mean age, 58.4 vs. 53.6 and 47.6 years, respectively; both P < 0.001) and a higher proportion of patients with RA were female compared with patients with PsA or axSpA (77.2 vs. 52.5% and 36.3%, respectively; P < 0.001). Patients with PsA had a higher BMI (mean, 31.6 vs. 30.3 kg/m2; P < 0.001), and a higher proportion had obesity (53.3 vs. 44.6%; P < 0.001 for overall distribution of BMI categories) compared with those with RA, whereas patients with axSpA had comparable BMI (mean, 29.8 kg/m2) and prevalence of obesity (41.7%) relative to patients with RA. Overall, 14.1% of patients with RA, 9.4% with PsA, and 13.5% with axSpA were disabled. A lower proportion of patients with RA were employed full time (36.8%), and a higher proportion were retired (32.6%) compared with those with PsA (54.5 and 21.1%, respectively) or axSpA (60.6 and 11.8%, respectively) (P < 0.001 for overall distribution of work status).
Clinical Characteristics and Treatment History
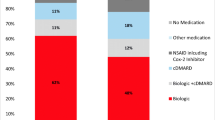
At the time of enrollment, patients with RA had shorter symptom duration (mean, 9.4 years) and disease duration (7.6 years) than those with PsA (11.2 and 8.4 years, respectively) or axSpA (16.7 and 9.8 years, respectively) (all P < 0.001) (Fig. 1). Patient comorbidity and treatment history at the time of registry enrollment are summarized in Table 2. Higher proportions of patients with PsA or axSpA had prior biologic use (27.3 and 30.7% vs. 23.3%; P < 0.001 for both) and were receiving biologics at enrollment (59.2 and 65.5% vs. 41.3%; P < 0.001 for both) compared with those with RA, whereas a higher proportion of patients with RA had prior csDMARD use and were receiving csDMARDs at enrollment compared with those with PsA or axSpA (prior csDMARD use, 29.6 vs. 25.0% and 18.4%; P < 0.001 for both; current csDMARD use, 79.9 vs. 53.2% and 20.8%; P < 0.001 for both). Additionally, higher proportions of patients with RA had a history of prednisone use (40.4%) and were currently using prednisone at enrollment (30.3%) compared with those with PsA (13.4 and 7.4%, respectively) or axSpA (11.5 and 6.1%, respectively) (all P < 0.001).
Disease Activity and PRO Measures
Patients with RA had higher TJC28 (mean, 4.7) and SJC28 (3.4) than those with PsA (2.9 and 1.8, respectively) or axSpA (2.4 and 1.8, respectively) (all P < 0.001) (Table 3). Patients with RA also had higher CDAI (mean, 14.4 vs. 12.1; P = 0.036), modified DAS28 (3.7 vs. 3.5; P = 0.001), and physician global assessment (27.3 vs. 18.6; P < 0.001) scores than those with PsA; scores among patients with axSpA were comparable to those of patients with RA (CDAI, 13.4; modified DAS28, 3.6; physician global assessment, 25.5) (Table 3 and Fig. 2).
Baseline scores among patients with RA, PsA, or axSpA for a physician global assessment, b patient global assessment, c patient-reported pain, and d patient-reported fatigue. *P < 0.05 and †P < 0.001 for comparison between patients with RA vs. PsA or RA vs. axSpA. Significance was consistent between unadjusted models and in general linear models adjusted for disease and symptom duration. AxSpA axial spondyloarthritis, PsA psoriatic arthritis, RA rheumatoid arthritis, VAS visual analog scale
With respect to PROs, patients with axSpA had higher pain (mean, 46.1 vs. 39.5; P < 0.001), fatigue (48.3 vs. 43.4; P = 0.001), and patient global assessment (50.2 vs. 36.9; P < 0.001) scores than those with RA, whereas patients with PsA had a comparable pain score (38.9 vs. 39.5; P = 0.451), lower fatigue score (41.1 vs. 43.4; P = 0.008), and higher patient global assessment score (43.2 vs. 36.9; P < 0.001) than those with RA (Fig. 2). Higher proportions of patients with PsA or axSpA reported experiencing ≥ 30 min of morning stiffness compared with those with RA (64.6 and 71.1% vs. 62.1%; P = 0.043 and P < 0.001, respectively) (Table 3). Patients with RA had a higher HAQ score than those with PsA or axSpA (mean, 0.9 vs. 0.6 and 0.7, respectively; both P < 0.001); patients with RA also had a higher modified HAQ score than those with PsA (0.4 vs. 0.3, respectively; P < 0.001) (Table 3).
Discussion
Rheumatoid arthritis, PsA, and axSpA share several clinical features, including joint inflammation and destruction, pain, reduced functional ability, and increased risk for comorbidities. TNF is an important inflammatory mediator in all three diseases, and TNFis have traditionally been used as first-line biologic therapy for patients with these conditions. Because of the similarities in symptoms and therapeutic choices, healthcare providers and payers often do not differentiate patients with RA, PsA, or axSpA with respect to disease management. However, recent trials of therapies targeting other inflammatory mediators have identified key differences in the underlying pathogenesis of these diseases, which has led to the approval of novel therapies that target cytokines specifically relevant to each disease, including IL-6 inhibitors for the treatment of RA and IL-17 and IL-12/23 inhibitors for PsA and axSpA. To ensure prompt, accurate diagnosis and facilitate treatment choices in the expanding therapeutic landscape, a thorough understanding of the differences in symptom presentation and disease burden of RA, PsA, and axSpA from both clinical and patient perspectives is necessary.
Time from symptom onset to diagnosis was longer in patients with axSpA (≈ 7 years) than in patients with RA (≈ 2 years) or PsA (≈ 3 years). This result indicates a substantial delay in diagnosis of axSpA and highlights the invisible nature of this disease. Factors contributing to the diagnostic delay of axSpA include the common occurrence of back pain in the general population and the lack of clinical signs and symptoms specific to axSpA [16]. An estimated 20–30% of patients with axSpA will develop structural changes in the sacroiliac joints within the first 2 years of disease onset, and up to 60% of patients may develop spinal changes within the first 10 years [14]. Failure to diagnose axSpA in the early stages of disease results in delayed treatment and may therefore lead to worse patient outcomes. Our results also suggest that diagnosis of PsA may be delayed compared with diagnosis of RA. The heterogeneity of PsA symptoms and the overlap of PsA symptoms with symptoms of other inflammatory rheumatic diseases may lead to misdiagnosis and contribute to this delay [11]. A delay in PsA diagnosis of > 6 months can lead to increased peripheral joint damage and functional disability [36]; early diagnosis and treatment are therefore critical to improve patient outcomes. Increased clinical education, the development and validation of clinically feasible disease activity and PRO measures, and improved clinical attention to PsA- and axSpA-related comorbidities have been identified as current unmet needs to enhance early diagnosis and management of these diseases [37].
In our study population, patients with PsA or axSpA had a disease burden comparable to or greater than patients with RA when assessed using common PRO measures; however, patient perspectives on disease burden were not reflected in clinical measures of disease activity. Similar discordance between patient and physician perceptions of the burden of inflammatory rheumatic diseases has been observed in previous studies conducted in Europe. A cross-sectional study conducted at an outpatient clinic in Norway found that patients with PsA or axSpA reported higher pain, fatigue, and patient global assessment scores than patients with RA did, despite comparable physician global assessment scores [38]. Similarly, an analysis using data from the German Collaborative Arthritis Centres database found comparable or worse health-related quality of life in patients with PsA or AS relative to patients with RA in terms of pain, functional ability, global health, and HAQ scores; however, a higher proportion of patients with RA were identified as having high disease activity by their physicians compared with patients with PsA or axSpA [39]. A study comparing physician and patient global assessment scores among physicians participating in the DANBIO registry and their patients with RA, PsA, or axSpA found that in approximately half of clinical encounters, patient global assessment scores were more than 20 mm higher (using a 100-mm VAS scale) than physician global assessment scores; the overall rate of discordance was highest among patients with PsA (56.5% of encounters vs. 49.0% in patients with RA and 48.9% in patients with axSpA) [40]. The differences in patient-reported vs. physician-reported measures observed in our study suggest that patients with PsA or axSpA perceive a greater disease burden than is recognized by their treating physicians, providing evidence of discordance between patient and physician perceptions of disease burden in a US-based setting.
Previously, tools for the clinical assessment of disease activity in patients with PsA or axSpA were adapted from those used in patients with RA. However, instruments that function well for RA may not provide a comprehensive picture of disease activity in PsA or axSpA. For example, the reduced 28-joint counts developed and validated for RA [41] may be used in clinical practice to save time compared with the TJC68 and SJC66; however, the reduced 28-joint counts may not detect up to 10% of patients with active PsA, particularly those with oligoarticular disease [42]. Additionally, the CDAI and DAS28, which were developed and validated for RA, are composite disease activity measures focused on articular manifestations and do not account for the heterogeneity of PsA and axSpA or manifestations such as axial skeletal disorders, enthesitis, and nail or skin changes [41, 43]. Recently, composite disease activity measures specific to PsA and axSpA have been developed to better assess disease activity, including the Disease Activity Index for Psoriatic Arthritis, Psoriatic Arthritis Disease Activity Score, Ankylosing Spondylitis Disease Activity Score, and Bath Ankylosing Spondylitis Disease Activity Index; these measures better reflect the unique features and extra-articular manifestations of PsA and axSpA and are recommended for clinical assessment of these diseases [44]. The results of our study indicate that the impact of PsA and axSpA may be underestimated by RA disease activity measures and highlight the importance of disease-specific assessments for determining clinical disease burden.
This study is subject to the general limitations of real-world observational studies. Patients enrolled in registries may not be representative of patients seen elsewhere in general practice. Patients in this study are routinely seen and treated by rheumatologists voluntarily participating in the Corrona RA and PsA/SpA registries; these patients may not be representative of all patients with RA, PsA, or axSpA in the United States, many of whom are not being treated by a rheumatologist. No statistical comparisons were performed for patient demographics, clinical characteristics, disease activity measures, or PRO measures between patients with PsA and those with axSpA; thus, no conclusions can be drawn regarding disease burden in patients with PsA vs. axSpA. All comparisons were descriptive; no adjustments were made to account for differences in patient characteristics such as age and sex; these and other characteristics may have influenced the other differences observed among the disease states. For example, patients with RA were significantly older than patients with PsA or axSpA, which may have influenced the differences in work status and presence of comorbidities among the patient populations. Additionally, patients with RA were predominantly female, whereas patients with axSpA were predominantly male, and the PsA population was generally balanced with respect to sex. Previous studies have reported higher pain scores and lower scores in physical and global health domains of health-related quality-of-life assessments in female vs. male patients with inflammatory rheumatic diseases [38, 39, 45,46,47]. Comparisons were limited to disease measures captured in Corrona that are directly comparable across all three disease states; due to the limited number of these measures, the data collected in this study may not provide a comprehensive picture of the impact of RA, PsA, and axSpA on patients’ lives.
Conclusions
In this real-world US patient population, patients with PsA or axSpA had a significantly longer time from symptom onset to diagnosis than patients with RA did, suggesting that PsA and axSpA may not be as well recognized in clinical practice compared with RA. Patients with PsA or axSpA had disease burden comparable to or greater than that in patients with RA when assessed using common PRO measures; however, disease burden in patients with PsA or axSpA appeared lower when assessed using RA disease activity measures. Overall, these results provide physicians with important insights into the impact of RA, PsA, and axSpA and highlight the need for disease-specific clinical measures and management strategies to optimize disease control in patients with PsA or axSpA.
References
Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004–2014. Rheumatol Int. 2017;37:1551–7.
Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–108.
Rindfleisch JA, Muller D. Diagnosis and management of rheumatoid arthritis. Am Fam Phys. 2005;72:1037–47.
Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81.
Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512–6.
Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69:729–35.
Myers WA, Gottlieb AB, Mease P. Psoriasis and psoriatic arthritis: clinical features and disease mechanisms. Clin Dermatol. 2006;24:438–47.
Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64:14–7.
Gladman DD. Clinical features and diagnostic considerations in psoriatic arthritis. Rheum Dis Clin North Am. 2015;41:569–79.
Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060–71.
Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014;74:423–41.
Rudwaleit M, van der Heijde D, Landewe R, et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–83.
Rudwaleit M, van der Heijde D, Landewe R, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70:25–31.
Sieper J, Braun J, Dougados M, Baeten D. Axial spondyloarthritis. Nat Rev Dis Primers. 2015;1:15013.
Deodhar A, Mease PJ, Reveille JD, et al. Frequency of axial spondyloarthritis diagnosis among patients seen by United States rheumatologists for evaluation of chronic back pain. Arthritis Rheumatol. 2016;68:1669–76.
Deodhar A, Mittal M, Reilly P, et al. Ankylosing spondylitis diagnosis in US patients with back pain: identifying providers involved and factors associated with rheumatology referral delay. Clin Rheumatol. 2016;35:1769–76.
van der Heijde D, Sieper J, Elewaut D, Deodhar A, Pangan AL, Dorr AP. Referral patterns, diagnosis, and disease management of patients with axial spondyloarthritis: results of an international survey. J Clin Rheumatol. 2014;20:411–7.
Rusman T, van Vollenhoven RF, van der Horst-Bruinsma IE. Gender differences in axial spondyloarthritis: women are not so lucky. Curr Rheumatol Rep. 2018;20:35,018-0744-2.
Erol K, Gok K, Cengiz G, Kilic G, Kilic E, Ozgocmen S. Extra-articular manifestations and burden of disease in patients with radiographic and non-radiographic axial spondyloarthritis. Acta Reumatol Port. 2018;43:32–9.
van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978–91.
Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26.
Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960–77.
Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet. 2018;391:2273–84.
Gudu T, Gossec L. Quality of life in psoriatic arthritis. Expert Rev Clin Immunol. 2018;14:405–17.
Strand V, Khanna D. The impact of rheumatoid arthritis and treatment on patients’ lives. Clin Exp Rheumatol. 2010;28:S32–40.
Strand V, Singh JA. Patient burden of axial spondyloarthritis. J Clin Rheumatol. 2017;23:383–91.
Wan SW, He HG, Mak A, et al. Health-related quality of life and its predictors among patients with rheumatoid arthritis. Appl Nurs Res. 2016;30:176–83.
Michaud K, Wolfe F. Comorbidities in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:885–906.
Husted JA, Thavaneswaran A, Chandran V, et al. Cardiovascular and other comorbidities in patients with psoriatic arthritis: a comparison with patients with psoriasis. Arthritis Care Res. (Hoboken). 2011;63:1729–35.
Khraishi M, MacDonald D, Rampakakis E, Vaillancourt J, Sampalis JS. Prevalence of patient-reported comorbidities in early and established psoriatic arthritis cohorts. Clin Rheumatol. 2011;30:877–85.
van der Horst-Bruinsma IE, Nurmohamed MT, Landewe RB. Comorbidities in patients with spondyloarthritis. Rheum Dis Clin North Am. 2012;38:523–38.
Gherghe AM, Dougados M, Combe B, et al. Cardiovascular and selected comorbidities in early arthritis and early spondyloarthritis, a comparative study: results from the ESPOIR and DESIR cohorts. RMD Open. 2015;1(e000128):2015-000128.
Lee S, Mendelsohn A, Sarnes E. The burden of psoriatic arthritis: a literature review from a global health systems perspective. P T. 2010;35:680–9.
Kremer JM. The CORRONA database. Clin Exp Rheumatol. 2005;23:S172–7.
Kremer JM. The Corrona US registry of rheumatic and autoimmune diseases. Clin Exp Rheumatol. 2016;34:S96–9.
Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045–50.
Winthrop KL, Strand V, van der Heijde D, et al. The unmet need in rheumatology: reports from the targeted therapies meeting 2017. Clin Immunol. 2018;186:87–93.
Michelsen B, Fiane R, Diamantopoulos AP, et al. A comparison of disease burden in rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis. PLoS One. 2015;10:e0123582.
Zink A, Thiele K, Huscher D, et al. Healthcare and burden of disease in psoriatic arthritis. A comparison with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 2006;33:86–90.
Lindström Egholm C, Krogh NS, Pincus T, et al. Discordance of global assessments by patient and physician is higher in female than in male patients regardless of the physician’s sex: data on patients with rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis from the DANBIO registry. J Rheumatol. 2015;42:1781–5.
van Gestel AM, Haagsma CJ, van Riel PL. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998;41:1845–50.
Coates LC, FitzGerald O, Gladman DD, et al. Reduced joint counts misclassify patients with oligoarticular psoriatic arthritis and miss significant numbers of patients with active disease. Arthritis Rheum. 2013;65:1504–9.
Aletaha D, Nell VP, Stamm T, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7:R796–806.
Smolen JS, Schols M, Braun J, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis. 2018;77:3–17.
Barnabe C, Bessette L, Flanagan C, et al. Sex differences in pain scores and localization in inflammatory arthritis: a systematic review and metaanalysis. J Rheumatol. 2012;39:1221–30.
Dagfinrud H, Mengshoel AM, Hagen KB, Loge JH, Kvien TK. Health status of patients with ankylosing spondylitis: a comparison with the general population. Ann Rheum Dis. 2004;63:1605–10.
Salaffi F, Carotti M, Gasparini S, Intorcia M, Grassi W. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual Life Outcomes. 2009;7:25,7525-7-25.
Acknowledgements
The authors thank the participating providers and patients for contributing data to the Corrona RA and PsA/SpA registries.
Funding
This study was sponsored by Corrona, LLC. Corrona has been supported through contracted subscriptions in the last 2 years by AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Crescendo, Eli Lilly and Company, Genentech, Gilead, GSK, Janssen, Merck, Momenta Pharmaceuticals, Novartis, Ortho Dermatologics, Pfizer Inc., Regeneron, Roche, Sun, and UCB. The design and conduct of the study were a collaborative effort between Corrona, LLC, and Novartis, and financial support for the study was provided by Novartis. Novartis participated in the interpretation of data and review and approval of the manuscript. The Rapid Service Fee were funded by Novartis.
Medical Writing and Editorial Assistance
Support for third-party writing assistance for this manuscript, furnished by Elizabeth Ohneck, PhD, of Health Interactions, Inc, was provided by Novartis Pharmaceuticals Corporation, East Hanover, NJ.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Philip J. Mease has received research grants from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Lilly, Novartis, Pfizer, and UCB; consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Corrona, Galapagos, Gilead, Janssen, Lilly, Merck, Novartis, Pfizer, Sun, and UCB; and speakers bureau fees from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Genentech, Janssen, Lilly, Novartis, Pfizer, and UCB. Mei Liu is an employee of Corrona, LLC. Sabrina Rebello is an employee of Corrona, LLC. Hyungjoo Kang is an employee of Corrona, LLC. Esther Yi Park is an employee of Novartis Pharmaceuticals Corporation. Yujin Park is an employee of Novartis Pharmaceuticals Corporation. Jeffrey D. Greenberg is an employee and shareholder of Corrona, LLC, and has received consulting fees from Eli Lilly, Genentech, Janssen, Novartis, and Pfizer.
Compliance with Ethics Guidelines
The Corrona RA and PsA/SpA registries and their investigators have been reviewed and approved by a central institutional review board (IRB; New England Independent Review Board No. 120160610 [RA] and No. 120160070 [PsA/SpA]). For academic investigative sites that did not receive a waiver to use the central IRB, full board approval was obtained from the respective governing IRBs. All research was conducted in compliance with the Declaration of Helsinki of 1964 and all later amendments. All registry participants were required to provide written informed consent and authorization prior to participating.
Data Availability
The data sets generated and analyzed during the current study are not publicly available because of proprietary rights but are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.9725150.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Mease, P.J., Liu, M., Rebello, S. et al. Comparative Disease Burden in Patients with Rheumatoid Arthritis, Psoriatic Arthritis, or Axial Spondyloarthritis: Data from Two Corrona Registries. Rheumatol Ther 6, 529–542 (2019). https://doi.org/10.1007/s40744-019-00172-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-019-00172-9