Abstract
Introduction
The objective of this systematic review and meta-analysis is to systematically identify and review the efficacy of pharmacological treatments in men with osteoporosis.
Methods
Medline (via Ovid) and Cochrane CENTRAL were searched up to May 2023 for any randomized controlled trial (RCT) evaluating the efficacy of osteoporotic treatment on the evolution of Bone Mineral Density (BMD) and incidence of fractures of men suffering from primary osteoporosis. If at least two studies used the same pharmacological treatment and evaluated the same outcome, a random effect model meta-analysis was applied to reported pooled mean difference (MD) and 95% confidence interval (CI).
Results
From the 1,061 studies identified through bibliographic search, 21 RCTs fitted the inclusion criteria. Bisphosphonates (k = 10, n = 2992 men with osteoporosis) improved all three BMD sites compared to placebo; lumbar spine: MD + 4.75% (95% CI 3.45, 6.05); total hip: MD + 2.72% (95% CI 2.06; 3.37); femoral neck: MD + 2.26% (95% CI 1.67; 2.85). Denososumab (k = 2, n = 242), Teriparatide (k = 2, n = 309) and Abaloparatide (k = 2, n = 248) also produced significant improvement of all sites BMD compared to placebo. Romosozumab was only identified in one study and was therefore not meta-analysed. In this study, Romosozumab increased significantly BMD compared to placebo. Incident fractures were reported in 16 RCTs but only four reported fractures as the primary outcome. Treatments were associated with a lower incidence of fractures.
Conclusions
Medications used in the management of osteoporosis in women appear to provide similar benefits in men with osteoporosis. Therefore, the algorithm for the management of osteoporosis in men could be similar to the one previously recommended for the management of osteoporosis in women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue leading to increased bone fragility and fracture susceptibility [1]. Bone fractures are a major health consequence of osteoporosis leading to an increased risk of mortality, disability, loss of independence and increased medical costs [2,3,4]. Worldwide, 23% of women and 12% of men have osteoporosis, with the prevalence increasing significantly with age [5]. At the age of 50, the lifetime risk of experiencing a fracture is about 20% for a man whilst it is close to 50% for a woman [6]. As compared with a woman, fragility fractures in men are associated with more morbidities, greater need for long-term care, more disabilities and higher mortality [7, 8]. Thus, osteoporosis in men represents a significant public health threat [9]. For example, in the European Union, the total health burden due to fractures in men in 2010 was estimated to be 384,000 lost quality-adjusted life years (QALYs) and projected to increase to 491,000 QALYs lost in 2025 [10].
Numerous pharmacological therapies have been proposed to reduce fracture risk in patients with osteoporosis. In a recent network meta-analysis including 108,797 individuals from 79 randomized controlled trials, Shen et al. reported that pharmacological therapies like alendronate zolendronate, risedronate, ibandronate, denosumab, abaloparatide, teriparatide and romosozumab were all effective treatments to reduce the risk of fractures [11]. The majority (i.e. 86%) of the trials included in this meta-synthesis sampled postmenopausal women. As a considerable amount of evidence is convergent regarding the efficacy of the above-mentioned therapies in women [11,12,13,14,15], it is now accepted from regulatory agencies to grant marketing authorization of these drugs for men with osteoporosis following the conduct of bridging studies [16, 17]. In these studies, the primary outcome is no longer the risk of fracture but rather an increase of bone mineral density (BMD) similar to that observed in women [18, 19]. Requirements for this bridging concept include the use of the same formulation, dose and route of administration; the inclusion of a male population with a fracture risk of a similar magnitude compared with that of the postmenopausal women studied; and demonstration of similar changes in BMD in a 1‐year study [16, 17].
Although the efficacy of pharmacological treatment has been less studied in men compared to women, some meta-research studies have been published in the last few years to summarize the available evidence in men [20]. In 2015, Chen et al. [21] published a network meta-analysis aiming to provide a hierarchy of eight different drugs for their impact on bone mineral density of men and included 13 randomized controlled trials published until 2014. The authors reported that zoledronate had the most significant effect on increasing lumbar spine bone mineral density followed by alendronate, the combination of teriparatide + risedronate, risedronate alone, teriparatide alone, strontium ranelate, ibandronate and parathyroid hormone. Later in 2017, Nayak et al. [22] included 22 studies published up to 2016 in a meta-analysis aimed at assessing the evidence for the efficacy of treatment to reduce osteoporotic fracture risk in men. The authors concluded that bisphosphonates are effective in reducing the risk of vertebral fractures in men with osteoporosis but acknowledged that studies in this area are still required to provide robust evidence.
As some new trials have been published in the last 7 years, there is a need to update these works in order to provide the most up-to-date evidence-based data on the efficacy of osteoporosis pharmacological treatments in men. Therefore, the objective of this systematic review and meta-analysis is to systematically identify and review the efficacy of osteoporosis interventions in men.
Methods
The proposed systematic review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) 2020 [23]. A protocol has been developed and published in both PROSPERO (ID 395481) and Open Science Framework (https://osf.io/wqy3n/).
The research question can be summarized using the PICOs format: P (Population): Male adults (> 18 years) with primary osteoporosis (i.e. age-related osteoporosis); I (Intervention) Any osteoporosis treatment; C: Placebo or other active drugs; Outcome: evolution of bone mineral density (BMD) in three sites; lumbar spine (LS), total hip (TH) and femoral neck (FN); incidence of vertebral (V) and non-vertebral (NV) fractures.
Literature search
Medline (via Ovid) and Cochrane CENTRAL databases were searched in May 2023 for any randomized controlled trial evaluating the efficacy of osteoporotic treatment on the evolution of BMD and incidence of fractures of men suffering from primary osteoporosis. For convenience of translation, the search was limited to English and French studies [24]. A combination of terms of Medical Subject Headings (MeSH) and keywords was used in the search strategy (the complete search strategies for both databases are available in Appendix A1).
Additionally, a manual search within the bibliography of relevant papers was performed in order to complete the bibliographic search. Forward references searching of included studies was also conducted using Web of Science to identify other research that has referenced any article of interest. Previous systematic reviews and meta-analyses on the same topic were also searched for backward/forward referencing. Clinical trial registries (www.clinicaltrial.gov) were also searched for potential unpublished studies and experts in the field were contacted to obtain their opinions about the search strategy and the included papers. Those experts were asked to provide any missing studies or grey literature they were aware of. Finally, industry members developing osteoporosis treatments were contacted in order to obtain unpublished data on their products.
The search results from the electronic sources and hand searching were imported into Covidence software for data management.
Study selection
All identified articles were screened for their eligibility by two independent reviewers (C.B., C.D. or S.S) first based on their titles and abstracts and second, based on their full texts. Inclusion criteria (Table 1) guided the study selection process. During both stages, disagreements were resolved by consensus.
Studies with secondary causes of osteoporosis (cancer-related, hypogonadism and corticosteroid-induced osteoporosis), studies published in other languages than French and English [24], not original studies (case reports, review, letters to the editors, conference abstracts, opinion pieces) and protocols were excluded.
Data extraction
Data were extracted by one independent reviewer according to a standardized data extraction form pretested on a sample of 4 studies. A second reviewer checked data extraction for accuracy. The following data were extracted: information related to the reference (author, year of publication, journal, funding, conflicts of interest), information related to the study design (design of intervention, groups, sample size, analysis per protocol or intention-to-treat), information related to the treatment (type of treatment, dose, route of administration, length of follow-up) and information related to the outcome (mean BMD, incidence of fracture in each groups). Authors of individual papers were contacted in case of any missing information.
Quality appraisal
The Revised Cochrane risk-of-bias tool for randomized trials (RoB2) was used to appraise the quality of individual studies [25]. This tool assesses 5 domains: randomisation process, deviation from intended interventions, missing outcome data, measurement of the outcome and selection of the reported results. A judgment per domain and an overall judgment or risk of bias was provided and studies were rated as having low risk of bias, some concerns or high risk of bias. When a study did not publish a protocol, domain 5 was automatically rated as “some concerns”. When a study was not double-blinded, domain 2 was automatically rated as “some concerns”.
Each study was evaluated independently by two reviewers. Disagreements were resolved by consensus or with the help of a third expert reviewer.
Grading the evidence
For all significant associations determined by meta-analyses, the evidence derived from RCTs was evaluated using the GRADE (Grading of Recommendations, Assessments, Development and Evaluation) assessment [26]. The evidence score started at high-quality evidence and was downgraded by one (i.e. moderate quality evidence), two (i.e. low quality evidence) or three levels (i.e. very low quality evidence) if one of the following pre-specified criteria was present: 1) Risk of bias (i.e. high risk of bias in more than 75% of the included studies; 2) Inconsistency (i.e. unexplained substantial heterogeneity I2 > 50%); 3) Indirectness (i.e. presence of factors that limit the generalizability of the results); 4) Imprecision (i.e. large 95%CI, recommendation altered if 95%CI represents the true effect), 5) Publication bias (i.e. small study effect p > 0.05 and significant impact on the estimator). Each meta-analysis outcome assessed was determined to be of very low, low, moderate or high certainty.
Data synthesis
Results on the effect of treatments on (1) BMD sites and (2) incidence of fractures were presented. Unfortunately, because fractures were rarely reported as primary endpoint in the included studies and mostly presented as safety results, it was not considered appropriate to perform a meta-analysis on this outcome. Results regarding the effect of fractures were therefore only presented in a narrative form.
For the effect on BMD, if at least two studies using the same pharmacological treatment and evaluating the same outcome were available to be pooled in a meta-analytical model, a random effect model was applied to calculate a pooled Mean Difference for each BMD site. Separate meta-analyses were performed for each treatment. Additionally, anti-resorptive and anabolic agents were grouped together and the pooled MD for both types of treatment were obtained by random effect model. When possible, effect sizes from intent-to-treat analysis were used in our analyses. For studies reporting the outcomes for multiple follow-up time periods, the outcomes reported for the longest follow-up time period under treatment was used. Results were examined for heterogeneity using Cochran’s Q statistic and the I2 statistic. The Egger's regression asymmetry test was used to detect publication bias for meta-analysis including a sufficient number of studies. One-way sensitivity analyses were also conducted to evaluate the stability of the results when one study is removed at a time.
When data were not available in the right format or incomplete, authors of individual studies were contacted to obtain missing values. Authors were contacted twice with a one-month interval between contacts. If the missing data could not be obtained from the authors, different strategies to obtain the missing information were used: (1) application of the methods described in Sect. 7.7.3 of the Cochrane Handbook for Systematic Review [27] to obtain missing SD’s from SE, from p-values or 95% confidence intervals; (2) when no information was provided to obtain the missing SD’s, SD’s were extracted from another study with a similar sample size, (3) when only median and interquartile ranges were available, the formula proposed by Hozo et al. [28] to convert them into mean and SDs was used.
For all results, a two-sided p value of 0.05 or less was considered as significant. All analyses were performed using R Software and appropriate packages.
If a meta-analysis could not be performed, a narrative description of the results was provided.
Results
Studies characteristics and risk of bias evaluation
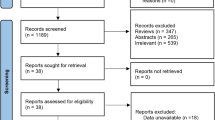
A total of 1,254 references were identified through the search strategies applied on bibliographic databases in May 2023 2022. After removing duplicates, 1,061 references were assessed for eligibility based on their title/abstract. Among those references, 104 were assessed based on their full text and 21 RCTs met the inclusion criteria and were further included in this systematic review and meta-analysis [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50] (Fig. 1). The list of excluded studies in the stage of full-text review as well as of the reason for exclusion is available on our Open Science Framework deposit (https://osf.io/wqy3n/).
Studies were published between 2003 and 2022. The number of male patients included in the studies varied from 20 in the study of Matsumoto et al. [51] to 1199 in the study of Boonen et al. [44]. Sixteen out of the 21 included RCTs (i.e. 76.2%) were double-blinded. The efficacy of 8 different treatments was investigated through those 21 RCTs: alendronate (n = 8), risedronate (n = 3), zoledronic acid (n = 3), ibandronate (n = 1), denosumab (n = 2), teriparatide (n = 5), abaloparatide (n = 2), romosozumab (n = 1). Most of the studies (n = 17, 81%) used placebo as comparator; other studies were head-to-head RCTs [35, 36, 41, 48] comparing the efficacy of two active drugs. The median length of treatment was 78 weeks (range 24 weeks [47] to 156 weeks [49]). In regards of outcomes, all studies reported measures of BMD (n = 21, 100%), with lumbar spine BMD defined as primary outcome in 14 studies (66.7%). Incidence of fracture was reported in 16 studies (76.2%) and only four studies defined the incidence of fracture as the primary outcome. In the other studies, incidence of fracture was reported either as a secondary outcome or as a safety measurement. Table 2 contains the detailed characteristics of the 21 RCTs included.
Only one study presented a high risk of bias [36]. However, Boonen et al. 2012 [44], Orwoll et al. 2012 [38], Matsumoto et al. [51] were the only three studies rated as low risk of bias studies. All the other studies presented some concerns in regard to the risk of bias and mainly for Domain 5 (i.e. selection of the reported results). Indeed, most of the studies (i.e. 14/21, 66.7%) did not published an a priori protocol (Appendix A2).
Due to the number of studies available, five different meta-analyses were possible: (1) effects of any bisphosphonate versus placebo on BMD; (2) effects of alendronate versus placebo on BMD; (3) effects of risedronate versus placebo on BMD; (4) effects of teriparatide versus placebo on BMD and (5) effects of abaloparatide versus placebo on BMD. All the other associations with BMD as well as all associations between treatments and incidence of fractures were described narratively.
Effect of bisphosphonates versus placebo on BMD
Any bisphosphonates (Alendronate, Risedronate, Ibandronate, Zoledronic Acid)
Ten studies, including 2992 men with osteoporosis, compared treatment with bisphosphonates to placebo [30, 32,33,34, 39, 40, 43, 44, 47, 49]. Meta-analytic synthesis was feasible for all investigated outcomes (LS BMD n = 9, TH BMD n = 9, FN BMD n = 8). Duration of treatment ranged from 6 months [47] in the study of Hwang et al. to three years in the study of Gonneli et al. [49].
Compared to placebo, bisphosphonates significantly increased BMD at 3 sites (Fig. 2): LS BMD (Mean Difference of 4.75% (95% CI 3.45, 6.05), I2 = 79%), TH BMD (Mean Difference of 2.72% (95% CI 2.06; 3.37), I2 = 41%) and FN BMD (Mean Difference of 2.26% (95% CI 1.67; 2.85), I2 = 31%). No significant publication bias was found in those three meta-analyses (Egger test p > 0.05).
The one-leave-out analysis (Appendix A3) did not revealed the specific impact of any of the individual study on the pooled effect size.
Alendronate
Five studies, including 553 men with osteoporosis, compared alendronate to placebo [32, 34, 40, 47, 49]. Three studies provided daily 10 mg alendronate administration during, respectively 1 year [40], 2 years [34] and 3 years [49]. Two others provided weekly 70 mg alendronate administration during either 6 months [47] or 1 year [32]. Meta-analysis was possible for all investigated outcomes (LS BMD n = 5, TH BMD n = 4, FN BMD n = 5, VF n = 3, NVF n = 3). Compared to placebo, alendronate seems to significantly improve LS BMD with a MD of 5.2% (95% CI 2.76;7.64) (I2 = 83%, p < 0.01), TH BMD with a MD of 2.34% (95% CI 1.66–3.03) (I2 = 0%, p = 0.81) as well as FN BMD with a MD of 2.53% (95% CI 1.76;3.31) (I2 26%, p = 0.25).
Risedronate
Two studies, including 600 men with osteoporosis treated during 2 years, compared risedronate to placebo [39, 43]. Boonen et al. provided once-weekly administration of 35 mg of risedronate or placebo whereas Ringe et al. provided daily administration of 5 mg of risedronate or placebo. Risedronate seems to be beneficial for all the investigate outcomes except the incidence of vertebral fractures (p = 0.25). A MD of 4.39% (95% CI 3.46;5.31) (I2 = 0%, p = 0.83) was found for LS BMD, a MD of 2.46% (95% CI 1.71;3.22) (I2 = 0%, p = 0.7) was found for TH BMD and a MD of 1.95% (95% CI 0.62;3.27) (I2 = 67%, p = 0.08) was found for FN BMD.
Zoledronic acid
Two studies, including 1,707 men with osteoporosis treated during 2-years, compared zoledronic acid to placebo [30, 44]. Because papers did not report similar outcomes, meta-analytic synthesis was not possible for zoledronic acid. In 2012, Boonen et al. 2012 [44] reported a significant improvement of LS BMD in 1,199 patients treated with yearly intravenous injection of 5 mg Zoledronic Acid (MD of 6.10%, 95% CI 4.99–7.21). A significant improvement of TH BMD (difference between groups of 3.8%, 95%CI 2.2–5.4) and FN BMD (difference between group of 3.1%, 95% CI 2.2; 5.4) was also demonstrated in a sample of 508 men with a recent hip fracture (Boonen et al. 2011 [30]).
Ibandronate
One study, including 132 men with osteoporosis treated for 1 year, compared ibandronate (150 mg, monthly) to placebo [33]. In this study, Orwoll et al. [33] reported a significant improvement of LS BMD (difference between groups of 2.58%, 95% CI 1.41; 3.76) and TH BMD (difference between groups of 2.13%, 95% CI 1.34; 2.92) in the ibandronate group compared to the placebo group.
Effects of other treatments versus placebo on BMD
Denosumab
Two RCTs, including 242 men with osteoporosis [38] and 47 men with osteoporosis respectively followed participants for two years [31] comparing denosumab to placebo. Both studies provided patients in the intervention arm with subcutaneous injection of 60 mg of Denosumab every 6 months. A significant improvement of all BMD sites was observed in the treated group compared to placebo. A MD of 5.80% (95% CI 3.5;8.1) (I2 = 78%, p = 0.03) was found for LS BMD, a MD of 2.28% (95% CI 1.51;3.04) (I2 = 16%, p = 0.27) was found for TH BMD and a MD of 2.07% (95% CI 1.23;2.92) (I2 = 0%, p = 0.85) for FN BMD (Appendix A4).
Teriparatide
Two studies, including 309 men with osteoporosis, compared teriparatide treatment to placebo [37, 45]. In the study of Orwoll et al. [37], patients were randomized to receive either daily subcutaneous injection of 20 µg of teriparatide (n = 151) or placebo (n = 147) (a third group receiving 40 µg of teriparatide (n = 139) was also included in the study but not used in our analyses as it is an uncommon dose of treatment) over two years. However, he study was stopped after 11 months. In the study of Kurland et al. [45], patients were randomized to receive either teriparatide (400 IU of parathyroid hormone acid fragment of human PTH (1–34)) (n = 13) or placebo (n = 10) during 18 months. Using meta-analytic statistics, a significant increase of LS BMD was found with teriparatide (MD 8.19, 95% CI 1.14;15.25) as well as a significant increase of FN BMD (MD 1.33, 95% CI 0.39;2.27) (Appendix A5). TH BMD was only reported in the study of Orwoll et al. and authors reported non-significant effect on TH BMD (+ 1.17% for teriparatide group vs + 0.54% for the placebo group, p = NS).
Abaloparatide
Two recent studies, including 248 men with osteoporosis, compared abaloparatide (80 µg daily) to placebo [42, 51] administered either during 1-year [42] or 18 months[51]. A significant improvement of all BMD sites was observed in the treated group compared to placebo. A MD of 11.29% (95% CI 1.80; 20.8) (I2 = 77%, p = 0.04) was found for LS BMD, a MD of 3.91% (95% CI 0.34; 7.49) (I2 = 95%, p < 0.01) for TH BMD and a MD of 3.98% (95% CI 1.10; 6.85) (I2 = 69%, p = 0.07) was found for FN BMD (Appendix A6).
Romosozumab
One study, including 245 men with osteoporosis compared romosozumab treatment to placebo during 12 months [29]. A monthly injection of 210 mg of romosozumab was provided to 163 men compared to placebo for 82 men. Results reported a mean percentage change from baseline in the LS BMD, TH and FN BMD significantly greater for the romosozumab group compared to the placebo group (LS, + 12.1% vs + 1.2%; TH, + 2.5% vs − 0.5%; FN, + 2.2% vs − 0.2%; all p < 0.001).
Head-to-head comparisons and effects on BMD
Four studies provided results of head-to-head comparisons. In two of them, teriparatide was compared to alendronate [36, 48], another compared teriparatide to risedronate [41] and the last compared alendronate to zoledronic acid [35].
In both studies comparing teriparatide (20 µg daily in the study of Qi et al. [36] and 40 µg daily in the study of Finkelstein et al. [48]) to alendronate (10 mg daily), authors reported a higher increase of LS BMD in the teriparatide group compared to alendronate (meta-analysis not feasible due to lack of quantitative data available). Finkelstein et al. [48] also measured impact of treatments on FN BMD and reported a significantly higher improvement of FN BMD for the teriparatide group as well.
In the study of Walker et al. [41], comparing a 18-month treatment with 35 mg weekly of risedronate (n = 10) to 20 µg daily of teriparatide (n = 9) (or a combination of both, n = 10), a significant increase of LS BMD was only noticed after the 18-month intervention. However, no significant difference between groups was mentioned. TH and FN BMD only increase significantly in the combination group after 18 months and this increase was significantly superior than the two other groups (p < 0.05).
In the study of Orwoll et al. [35], comparing a 2-year treatment with 5 mg yearly of zoledronic acid (n = 148) to 70 mg daily of alendronate (n = 158), authors reported an increase of LS BMD, TH BMD and FN BMD in both groups over 24-months. The noninferiority of zoledronic acid versus alendronate was established but superiority was not demonstrated.
Effects of treatments on fractures
Incidence of fractures (i.e. either vertebral or non-vertebral fractures) was reported in 16 studies (76%), but only four studies defined incidence of fracture as the primary outcome [30, 31, 39, 44]. In the study of Boonen et al. 2012 [44] including 1199 men with osteoporosis, authors observed that zoledronic acid provided during 12 months significantly reduced the incidence of fractures observed during 24-months of follow-up compared to placebo. The rate of any new morphometric vertebral fracture was 1.6% in the zoledronic acid group and 4.9% in the placebo group over the 24-month period, representing a 67% risk reduction with zoledronic acid (relative risk, 0.33; 95% confidence interval, 0.16 to 0.70; P = 0.002). In another study of Boonen et al. 2011 [30], a sample of 508 men with recent hip fracture was also randomized to either zoledronic acid or placebo. No significant difference between groups was found in regards of the rate of clinical fracture at month 24 (i.e. 7.5% in the zoledronic acid group vs 8.7% in the placebo group, p = 0.64). In the third study including 47 men with osteoporosis, the one of Nakamura et al. [31], authors reported no new vertebral fracture in men treated with denosumab versus an incidence of 8.3% in the placebo group. The difference between groups was not significant (p = 0.15). The fourth study, published by Ringe et al. 2009 [39] included 158 men with risedronate treatment during 2 years and 158 men with placebo. Authors reported a significantly higher incidence of fractures in the placebo group compared to the risedronate group (i.e. incidence of VF fractures of 23.6% versus 9.2%, p = 0.003; incidence of NVF of 22.3% versus 11.8%, p = 0.03). All other studies were not powered for this outcome. In most of included studies, authors simply reported the number of fractures in each group, without any statistical comparisons between them. Incidence of fractures was generally very low (median of VF 1.7% in the treatment group versus 4.1% in the PBO group across studies; median of NVF 3.8% in both groups across studies). None of the 16 included studies reported a higher risk of fractures in the treatment group compared to placebo. No safety issue in regards of the risk of fracture was therefore reported for any of the treatments.
Results of incidence of fracture within each of the 16 studies are reported in Table 3.
GRADE assessment
GRADE assessments is available in Appendix A7. GRADE level of evidence was attributed for all the 6 meta-analyses run (i.e. effects of any bisphosphonate versus placebo, alendronate versus placebo, risedronate versus placebo, denosumab vs placebo, teriparatide versus placebo and abaloparatide versus placebo on BMD). Risk of bias was considered as not serious in all studies included in those meta-analyses; publication bias was only measured in one meta-analysis run (i.e. any bisphosphonates versus placebo) because all other meta-analyses included a too restricted number of studies; inconsistency (i.e. unexplained heterogeneity) was observed for most of the meta-analyses; serious imprecision was considered for all meta-analysis comprising only 2 or 3 studies and no serious indirectness was considered for all meta-analyses. A high level of evidence was considered for the efficacy of any bisphosphonate on BMD ( ), a moderate level of evidence was considered for the efficacy of denosumab, alendronate and risedronate on BMD (
), a moderate level of evidence was considered for the efficacy of denosumab, alendronate and risedronate on BMD ( ) and, finaly, a low level of evidence was considered for the efficacy of teriparatide and abaloparatide on BMD (
) and, finaly, a low level of evidence was considered for the efficacy of teriparatide and abaloparatide on BMD ( ).
).
Discussion
This systematic review and meta-analysis provides evidence that alendronate, risedronate, zoledronic acid, ibandronate, denosumab, teriparatide abaloparatide and romosozumab all have a beneficial effect on lumbar spine, total hip and femoral neck BMD of men suffering from osteoporosis, as compared with placebo. The strength of evidence is however limited by the low number of studies included in the analyses and the unexplained heterogeneity observed in some comparisons. Fracture data in men are scant at all sites (vertebral, and non-vertebral fractures) and few randomized controlled studies have reported the efficacy of pharmacological treatment on the incidence of fracture as the primary endpoint. Hence, the efficacy of these treatments to reduce the incidence of fracture is still inconclusive. Nevertheless, as previously mentioned, regulatory agency guidance indicate that, once an indication in postmenopausal women as been granted, a separate bridging study based on changes in BMD can be sufficient to grant an authorization for men with osteoporosis [16, 17]. Bone mineral density is suggested as a “surrogate marker” in studies of men [18, 19], it is therefore not surprising that our systematic literature search identified a larger number of studies using BMD rather than fracture incidence as the primary outcome.
A previous systematic review and meta-analysis [21] also reported efficacy of osteoporosis treatment on BMD of men with osteoporosis. Authors included 13 randomized controlled studies published until 2014. Despite using slightly different inclusion criteria from ours and including a lower number of trials, they also confirmed the efficacy of most of the proposed treatment in improving BMD at the lumbar spine. Other BMD sites were not investigated. In the previous study, the authors also provided a hierarchy of treatments using network meta-analytic statistics. This methodology can however be discussed as the authors only included one study for the majority of comparisons investigated. This is the main reason why we decided not to perform a network meta-analysis on our data. Nevertheless, when comparing the effect sizes found in all of our analyses, abaloparatide seems to be the most effective treatment proposed for the increase of lumbar spine BMD. The effect size for abaloparatide (i.e. MD of 11.29) is superior to the effect size of teriparatide (i.e. MD of 8.19), which is in line with previous published data [12]. These assumptions are, however, only observational as providing a hierarchy between treatments was not the objective of the current study. Another systematic review and meta-analysis, published by Nayak et al. [22] reported efficacy of treatments on vertebral and non-vertebral fractures directly. Authors included 22 individual studies and revealed that alendronate and risedronate are effective in reducing vertebral fractures. However, they did not report significant effect of denosumab. One important point to highlight is, once again, that a very limited number of studies were included in each forest plot (e.g. only two studies for alendronate versus placebo and two studies for risedronate versus placebo comparisons). Pooling all bisphosphonates (4 studies respectively for vertebral and non-vertebral fractures risk association), the authors confirmed the beneficial effects of this treatment category to reduce the risk of both vertebral and non-vertebral fractures. This being said, our results are in line with those two previous meta-research studies as both of them demonstrated the efficacy of treatments either on BMD or on fracture risk.
Overall, our results also support current guidelines [1, 52, 53] to use bisphosphonates (alendronate, risedronate, ibandronate and zoledronate) [54] and denosumab in patients with high risk of fracture and teriparatide in patients with very high risk of fractures. Nevertheless, these guidelines have been developed for postmenopausal women with osteoporosis and specific guidelines for the management of men with high risk/very high risk of fractures may be developed by, among other strategies, taking the results of this present meta-analysis into consideration.
There are some strengths in this paper. Most importantly, we followed the best practices to conduct a systematic literature review and meta-analysis. As example, we used PRISMA2020 checklist [23] for the completeness of reporting, AMSTAR2 quality appraisal tool [55] to ensure a high-quality level to our methodology, Cochrane Handbook for systematic literature review and meta-analysis to guarantee a high-quality level to our meta-analytical statistics [27]. Also, we and used a comprehensive search strategy to minimize the possibility of publication bias. As a result of this, no publication bias was found in this systematic review and meta-analysis. This strategy was preferred to highlight the efficacy of treatment on osteoporosis alone. Our work nevertheless contains several limitations. First, a limited number of studies were included in the different forest plots, which prevented subgroup analyses exploring potential sources of heterogeneity, sensitivity analyses or publication bias analyses. This is particularly true for denosumab, abaloparatide and teriparatide for which only two randomized controlled trials met our inclusion criteria and were combined in a meta-analytic statistical model. For romosozumab, only one study was identified and no meta-analysis was run. Given the restricted number of studies available to measure the efficacy of these different treatments for osteoporosis in men, the strength of evidence is considered to be moderate to low. The second limitation of this work concerns the length of treatment that may vary from one study to another. In the forest plots, we combined studies that used various length of treatment, which is not optimal. Moreover, in the study of Hwang et al. patients were treated with alendronate during only 6 months. Even if participants improved their LS and FN BMD during this period, the non-significant effect on TH BMD could be partially explained by this short period of treatment. Third, it is regrettable that a very restricted number of studies used the incidence of fracture as primary outcome. In studies measuring the efficacy of pharmacological treatment on fracture incidence but defining fracture as a secondary outcome or a safety marker, the sample size and length of study may not be appropriate to detect a significant difference, if this exists. Therefore, it was considered inappropriate to run a meta-analysis on this outcome and the results were only presented narratively. Fourth, the majority of the clinical trials (i.e. 14 out of 21, 66.7%) included in this meta-analysis used the increase of lumbar spine bone mineral density as the primary outcome measure. Currently, studies aiming to validate change in bone mineral density as a surrogate endpoint for fracture outcomes mainly focused on hip bone mineral density. Stronger associations have been found between change in total hip BMD and incidence of vertebral and hip fractures [18, 19]. Nevertheless, these observations do not mean that using lumbar spine BMD increase as surrogate marker for fracture risk is irrelevant, as Bouxsein et al. [56] reported similar correlations values for vertebral fracture and change in lumbar spine, total hip and femoral neck BMD in a meta-regression including 38 placebo-controlled trials.
As previously mentioned, the European regulatory authorities accept the use of bone mineral density (BMD) as a primary outcome in pivotal studies evaluating the efficacy of chemical entities intended for the management of osteoporosis in males, providing these chemical entities were approved for the management of osteoporosis in females, based on a pivotal study showing a reduction in fracture rates. Total hip or femoral neck are widely recognized as the most appropriate measurement sites for BMD, in such studies (bridging studies). Nevertheless, a minimal duration of 12 months for bone forming agents and of 24 months for anti-resorptive agents is usually considered as a minimal length of treatment. In the future, the assessment of biochemical markers of bone remodeling could be further investigated to see whether CTX1 for anti-resorptive agents and PINP for bone-forming agents could also be considered as surrogate markers for anti-fracture efficacy, in the context of bridging studies.
Conclusion
Through a systematic review and meta-analysis including 21 randomized controlled trials, we have established that medications used in the management of osteoporosis in women (i.e. alendronate, risedronate, zoledronic acid, ibandronate, denosumab, teriparatide abaloparatide and romosozumab) appear to be similarly beneficial in men with osteoporosis. Therefore, the algorithm for the management of osteoporosis in men could be identical to that recommended for the management of osteoporosis in women.
Availability of data and materials
The databases and R script for running meta-analyses have been deposit on Open Science Framework (https://osf.io/wqy3n/).
References
Kanis JA, Cooper C, Rizzoli R et al (2019) Executive summary of European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Aging Clin Exp Res 31:15–17. https://doi.org/10.1007/s40520-018-1109-4
Sambrook P, Cooper C (2006) Osteoporosis. Lancet 367:2010–2018. https://doi.org/10.1016/S0140-6736(06)68891-0
Harvey N, Dennison E, Cooper C (2010) Osteoporosis: impact on health and economics. Nat Rev Rheumatol 6:99–105. https://doi.org/10.1038/nrrheum.2009.260
Bolland MJ, Grey AB, Gamble GD et al (2010) Effect of osteoporosis treatment on mortality: a meta-analysis. J Clin Endocrinol Metab 95:1174–1181. https://doi.org/10.1210/JC.2009-0852
Salari N, Ghasemi H, Mohammadi L et al (2021) The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J Orthop Surg Res 16:1–20. https://doi.org/10.1186/S13018-021-02772-0/FIGURES/8
Coughlan T, Dockery F (2014) CME geriatric medicine osteoporosis and fracture risk in older people. Clin Med 14:187–191
Causes BJ (2013) consequences, and treatment of osteoporosis in men. Drug Des Dev Ther. https://doi.org/10.2147/DDDT.S46101
Rinonapoli G, Ruggiero C, Meccariello L et al (2021) Osteoporosis in men: a review of an underestimated bone condition. Int J Mol Sci 22:2105. https://doi.org/10.3390/IJMS22042105
Kaufman JM (2021) Management of osteoporosis in older men. Aging Clin Exp Res 33:1439–1452
Hernlund E, Svedbom A, Ivergård M et al (2013) Osteoporosis in the European Union: Medical management, epidemiology and economic burden: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. https://doi.org/10.1007/s11657-013-0136-1
Shen J, Ke Z, Dong S et al (2022) Pharmacological therapies for osteoporosis: A Bayesian Network Meta-Analysis. Med Sci Monit 28:e935491–e935501. https://doi.org/10.12659/MSM.935491
Reginster JY, Bianic F, Campbell R et al (2019) Abaloparatide for risk reduction of nonvertebral and vertebral fractures in postmenopausal women with osteoporosis: a network meta-analysis. Osteoporos Int 30:1465. https://doi.org/10.1007/S00198-019-04947-2
Ellis AG, Reginster JY, Luo X et al (2014) Bazedoxifene versus oral bisphosphonates for the prevention of nonvertebral fractures in postmenopausal women with osteoporosis at higher risk of fracture: a network meta-analysis. Value Health 17:424. https://doi.org/10.1016/J.JVAL.2014.01.008
Migliorini F, Maffulli N, Colarossi G et al (2021) Effect of drugs on bone mineral density in postmenopausal osteoporosis: a Bayesian network meta-analysis. J Orthop Surg Res. https://doi.org/10.1186/S13018-021-02678-X
Ayers C, Kansagara D, Lazur B et al (2023) Effectiveness and safety of treatments to prevent fractures in people with low bone mass or primary osteoporosis: a living systematic review and network meta-analysis for the American College of Physicians. Ann Intern Med 176:182–195. https://doi.org/10.7326/M22-0684/SUPPL_FILE/M22-0684_SUPPLEMENT.PDF
US Food and Drug Administration. FDA Public Workshop. Osteoporosis Drug Development: Moving Forward. In: https://www.fda.gov/downloads/drugs/newsevents/ucm470574.pdf
European Medicines Agency. Guideline on the evaluation of medicinal products in the treatment of primary osteoporosis. In: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003405.pdf .
Black DM, Bauer DC, Vittinghoff E et al (2020) Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: meta-regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabet Endocrinol 8:672–682. https://doi.org/10.1016/S2213-8587(20)30159-5
Eastell R, Vittinghoff E, Lui L-Y et al (2021) Validation of the surrogate threshold effect for change in bone mineral density as a surrogate endpoint for fracture outcomes: the FNIH-ASBMR SABRE project. J Bone Min Res. https://doi.org/10.1002/jbmr.4433
Kaufman J-M, Reginster J-Y, Boonen S et al (2013) Treatment of osteoporosis in men. Bone 53:134–144. https://doi.org/10.1016/j.bone.2012.11.018
Chen LX, Zhou ZR, Li YL et al (2015) Comparison of bone mineral density in lumbar spine and fracture rate among eight drugs in treatments of osteoporosis in men: a network meta-analysis. PLoS ONE 10:e0128032. https://doi.org/10.1371/journal.pone.0128032
Nayak S, Greenspan SL (2017) Osteoporosis treatment efficacy for men: a systematic review and meta-analysis. J Am Geriatr Soc 65:490–495. https://doi.org/10.1111/jgs.14668
Page MJ, McKenzie JE, Bossuyt PM et al (2020) statement: an updated guideline for reporting systematic reviews. BMJ 2021:372. https://doi.org/10.1136/BMJ.N71
Morrison A, Polisena J, Husereau D et al (2012) The effect of english-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care 28:138–144. https://doi.org/10.1017/S0266462312000086
Higgins JPT, Altman DG, Gøtzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. https://doi.org/10.1136/BMJ.D5928
Brozek JL, Akl EA, Alonso-Coello P et al (2009) Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. an overview of the GRADE approach and grading quality of evidence about interventions. Allergy 64:669–677. https://doi.org/10.1111/j.1398-9995.2009.01973.x
Cochrane handbook for systematic reviews of interventions | Cochrane Training. [cited 13 Dec 2022]. Available: https://training.cochrane.org/handbook
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13. https://doi.org/10.1186/1471-2288-5-13
Michael Lewiecki E, Blicharski T, Goemaere S et al (2018) A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab 103:3183–3193. https://doi.org/10.1210/jc.2017-02163
Boonen S, Orwoll E, Magaziner J et al (2011) Once-yearly zoledronic acid in older men compared with women with recent hip fracture. J Am Geriatr Soc 59:2084–2090. https://doi.org/10.1111/j.1532-5415.2011.03666.x
Nakamura T, Matsumoto T, Sugimoto T et al (2014) Clinical trials express: fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT). J Clin Endocrinol Metab 99:2599–2607. https://doi.org/10.1210/jc.2013-4175
Miller PD, Schnitzer T, Emkey R et al (2004) Weekly oral alendronic acid in male osteoporosis. Clin Drug Invest 24:333
Orwoll ES, Binkley NC, Lewiecki EM et al (2010) Efficacy and safety of monthly ibandronate in men with low bone density. Bone 46:970–976. https://doi.org/10.1016/j.bone.2009.12.034
Orwoll E, Ark M, Ttinger E et al (2000) Alendronate for the treatment of osteoporosis in men. N Engl J Med 343:604–610
Orwoll ES, Miller PD, Adachi JD et al (2010) Efficacy and safety of a once-yearly i.v. infusion of zoledronic acid 5mg versus a once-weekly 70-mg oral alendronate in the treatment of male osteoporosis: a randomized, multicenter, double-blind, active-controlled study. J Bone Min Res 25:2239–2250. https://doi.org/10.1002/jbmr.119
Qi Y, Wang W, Sun W et al (2021) Comparative efficacy and safety of alendronate and teriparatide in bone loss reduction and prevention of vertebral fracture in osteoporotic Chinese patients. Trop J Pharm Res 20:2199–2204. https://doi.org/10.4314/tjpr.v20i10.26
Orwoll ES, Scheele WH, Paul S et al (2003) The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Min Res 18:9–17
Orwoll E, Teglbjærg CS, Langdahl BL et al (2012) A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab 97:3161–3169. https://doi.org/10.1210/jc.2012-1569
Ringe JD, Farahmand P, Faber H et al (2009) Sustained efficacy of risedronate in men with primary and secondary osteoporosis: Results of a 2-year study. Rheumatol Int 29:311–315. https://doi.org/10.1007/s00296-008-0689-2
Shimon I, Eshed V, Doolman R et al (2005) Alendronate for osteoporosis in men with androgen-repleted hypogonadism. Osteoporos Int 16:1591–1596. https://doi.org/10.1007/s00198-005-1879-3
Walker MD, Cusano NE, Sliney J et al (2013) Combination therapy with risedronate and teriparatide in male osteoporosis. Endocrine 44:237–246. https://doi.org/10.1007/s12020-012-9819-4
Czerwinski E, Cardona J, Plebanski R et al (2022) The efficacy and safety of abaloparatide-SC in men with osteoporosis: a randomized clinical trial. J Bone Miner Res. https://doi.org/10.1002/jbmr.4719
Boonen S, Orwoll ES, Wenderoth D et al (2009) Once-weekly risedronate in men with osteoporosis: results of a 2-Year, placebo-controlled, double-blind, multicenter study. J Bone Miner Res 24:719–725. https://doi.org/10.1359/jbmr.081214
Boonen S, Reginster J-Y, Kaufman J-M et al (2012) Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med 367:1714–1723. https://doi.org/10.1056/nejmoa1204061
Kurland ES, Cosman F, Mcmahon DJ et al (2000) Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab 85:3069
Zhou J, Liu B, Zhao QM et al (2020) Fall prevention and anti-osteoporosis in osteopenia patients of 80 years of age and older: a randomized controlled study. Orthop Surg 12:890–899. https://doi.org/10.1111/os.12701
Hwang JS, Liou MJ, Ho C et al (2010) The effects of weekly alendronate therapy in Taiwanese males with osteoporosis. J Bone Miner Metab 28:328–333. https://doi.org/10.1007/s00774-009-0136-9
Finkelstein JS, Hayes A, Hunzelman JL et al (2003) The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 349:1216
Gonnelli S, Cepollaro C, Montagnani A et al (2003) Alendronate treatment in men with primary osteoporosis: a three-year longitudinal study. Calcif Tissue Int 73:133–139. https://doi.org/10.1007/s00223-002-1085-7
Hou C, Li J, Wang X et al (2020) Comparison of efficacy and safety of teriparatide and hyaluronic acid-Calcitonin combination treatments in Chinese osteoporotic patients with risk of bone fracture: a preliminary investigation. Trop J Pharm Res 19:183–188. https://doi.org/10.4314/tjpr.v19i1.26
Matsumoto T, Sone T, Soen S et al (2022) Abaloparatide increases lumbar spine and hip BMD in Japanese patients with osteoporosis: the phase 3 ACTIVE-J study. J Clin Endocrinol Metab 107:e4222–e4231. https://doi.org/10.1210/clinem/dgac486
Kanis JA, Cooper C, Rizzoli R et al (2019) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 30:3–44. https://doi.org/10.1007/s00198-018-4704-5
Kanis JA, McCloskey EV, Harvey NC et al (2022) Intervention thresholds and diagnostic thresholds in the management of osteoporosis. Aging Clin Exp Res 34:3155. https://doi.org/10.1007/S40520-022-02216-7
Fuggle N, Al-Daghri N, Bock O et al (2022) Novel formulations of oral bisphosphonates in the treatment of osteoporosis. Aging Clin Exp Res 34:2625–2634. https://doi.org/10.1007/S40520-022-02272-Z
Shea BJ, Grimshaw JM, Wells GA et al (2007) Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 7:10. https://doi.org/10.1186/1471-2288-7-10
Bouxsein ML, Eastell R, Lui LY et al (2019) Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res 34:632–642. https://doi.org/10.1002/JBMR.3641
Funding
Open access funding provided by University of Geneva. ESCEO.
Author information
Authors and Affiliations
Contributions
JYR, RR, CC and OB conceived the study. Protocol was developed by CB and reviewed by all. Once the protocol was approved, CB. ran the search strategy. Study selection, data extraction and quality appraisal were done by CB, SS and CD. Statistical analyses were run by CB. GRADE assessment was done by CB and NV. First draft of the manuscript was written by CB. All authors read and approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
C.C. Cooper reports personal fees from Alliance for Better Bone Health, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfzer, Roche, Servier, Takeda and UCB. O.B. reports Grants or fees from Amgen, Aptissen, Biophytis, IBSA, Mylan, Novartis, Orifarm, Sanofi, UCB and Viatris. N.F. has received travel bursaries and speaker fees from Pfizer, Eli Lilly and Viatris. N.C.H. has received consultancy/lecture fees/honoraria/grant funding from Alliance for Better Bone Health, Amgen, MSD, Eli Lilly, Radius Health, Servier, Shire, Kyowa Kirin, UCB, Consilient Healthcare, Theramex and Internis Pharma. J-Y.R. reports Consulting fees or paid advisory boards for IBSA-GENEVRIER, MYLAN, RADIUS HEALTH, PIERRE FABRE, ECHOLIGHT, TEVA. Lecture fees when speaking at the invitation of sponsor: IBSA-GENEVRIER, MYLAN, CNIEL, DAIRY RESEARCH COUNCIL (DRC), TEVA. Grant Support from Industry (All through Institution): IBSA-GENEVRIER, MYLAN, CNIEL, RADIUS HEALTH. R.R. has received consulting fees from the advisory boards of Abiogen, Amgen, Danone, Echolight, European Milk Forum, Nestlé, ObsEva, Pfzer Consumer Health, Radius Health and Theramex. N.V. reports personal fees from MYLAN, FIDIA, IBSA, VIATRIS, BAYER, MSD, NESTLE', outside from this work. Other authors did not reported any conflict of interests.
Ethical approval/ Consent to participate/ Consent to publish
This is a systematic review. No ethical approval, consent to participate or consent to publish is required.
Statement of human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Beaudart, C., Demonceau, C., Sabico, S. et al. Efficacy of osteoporosis pharmacological treatments in men: a systematic review and meta-analysis. Aging Clin Exp Res 35, 1789–1806 (2023). https://doi.org/10.1007/s40520-023-02478-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-023-02478-9