Abstract
Objective
In view of the goal of eliminating tuberculosis (TB) by 2050, economic evaluations of interventions against the development of TB are increasingly requested. Little research has been published on the incremental cost effectiveness of preventative therapy (PT) in groups at high risk for progression from latent TB infection (LTBI) with Mycobacterium TB (MTB) to active disease. A systematic review of studies with a primary focus on model-driving inputs and methodological differences was conducted.
Methods
A search of MEDLINE, the Cochrane Library and EMBASE to July 2014 was undertaken, and reference lists of eligible articles and relevant reviews were examined.
Results
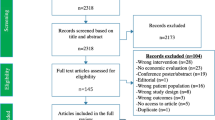
A total of 876 citations were retrieved, with a total of 24 studies being eligible for inclusion, addressing six high-risk groups other than contact persons. Results varied considerably between studies and countries, and also over time. Although the selected studies generally demonstrated cost effectiveness for PT in HIV-infected subjects and healthcare workers (HCWs), the outcome of these analyses can be questioned in light of recent epidemiologic data. For immigrants from high TB-burden countries, patients with end-stage renal disease, and the immunosuppressed, now defined as further vulnerable groups, no consistent recommendation can be taken from the literature with respect to cost effectiveness of screening and treating LTBI. When the concept of a fixed willingness-to-pay (WTP) threshold as a prerequisite for final categorization was used, the sums ranged between ‘no specification’ and US$100,000 per quality-adjusted life-year.
Conclusions
To date, incremental cost-effectiveness analyses on PT in groups at high risk for TB progression, other than contacts, are surprisingly scarce. The variation found between studies likely reflects variations in the major epidemiologic factors, particularly in the estimates on the accuracy of the tuberculin skin test (TST) and interferon-gamma release assays (IGRA) as screening methods used before considering PT. Further research, including explicit evaluation of local epidemiological conditions, test accuracy, and methodology of WTP thresholds, is needed.

Similar content being viewed by others
References
Diel R, Loddenkemper R, Zellweger JP, Sotgiu G, D’Ambrosio L, Forum European, Centis R, European Forum for TB Innovation, et al. Old ideas to innovate tuberculosis control: preventive treatment to achieve elimination. Eur Respir J. 2013;42:785–801.
Esmail H, Barry CE 3rd, Wilkinson RJ. Understanding latent tuberculosis: the key to improved diagnostic and novel treatment strategies. Drug Discov Today. 2012;17:514–21.
Stagg HR, Zenner D, Harris RJ, Muñoz L, Lipman MC, Abubakar I. Treatment of latent tuberculosis infection: a network meta-analysis. Ann Intern Med. 2014;161:419–28.
American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161:S221–47.
World Health Organization. Guidelines on the management of latent tuberculosis infection. WHO/HTM/TB/2015.01. Geneva: World Health Organization; 2015.
Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D, Pai M. The BCG world atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8:e100101249.
Nienhaus A, Schablon A, Costa JT, Diel R. Systematic review of cost and cost-effectiveness of different TB-screening strategies. BMC Health Serv Res. 2011;11:247.
Oxlade O, Pinto M, Trajman A, Menzies D. How methodologic differences affect results of economic analyses: a systematic review of interferon gamma release assays for the diagnosis of LTBI. PLoS One. 2013;8:e56044.
International Monetary Fund. Data and statistics. 2013. Available at: http://www.imf.org/external/data.htm. Accessed 3 Mar 2015.
Worldbank. Consumer price index (2010 = 100). 2014. Available at: http://data.worldbank.org/. Accessed 3 Mar 2015.
Akolo C, Adetifa I, Shepperd S, Volmink J. Akolo C, Adetifa I, et al. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010;(1):CD000171.
Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H; Centers for Disease Control and Prevention (CDC), National Institutes of Health, HIV Medicine Association of the Infectious Diseases Society of America. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58(RR-4):1.
Pape JW, Jean SS, Ho JL, Hafner A, Johnson WD Jr. Effect of isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection. Lancet. 1993;342:268–72.
Rose DN. Short-course prophylaxis against tuberculosis in HIV-infected persons: a decision and cost-effectiveness analysis. Ann Intern Med. 1998;129:779–86.
Centers for Disease Control and Prevention (CDC), American Thoracic Society. Update: adverse event data and revised American Thoracic Society/CDC recommendations against the use of rifampin and pyrazinamide for treatment of latent tuberculosis infection—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:735–9.
Whalen CC, Johnson JL, Okwera A, Horn DL, Huebner R, Mugyenyi P, et al. A trial of three regimens to prevent tuberculosis in Ugandan adults infected with the human immunodeficiency virus. Uganda-Case Western Reserve University Research Collaboration. N Engl J Med. 1997;337:801–8.
Bell JC, Rose DN, Sacks HS. Tuberculosis preventive therapy for HIV-infected people in sub-Saharan Africa is cost-effective. AIDS. 1999;13:1549–56.
Shrestha RK, Mugisha B, Bunnell R, Mermin J, Hitimana-Lukanika C, Odeke R, et al. Cost-effectiveness of including tuberculin skin testing in an IPT program for HIV-infected persons in Uganda. Int J Tuberc Lung Dis. 2006;10:656–62.
Shrestha RK, Mugisha B, Bunnell R, Mermin J, Odeke R, Madra P, et al. Cost-utility of tuberculosis prevention among HIV-infected adults in Kampala, Uganda. Int J Tuberc Lung Dis. 2007;11:747–54.
The World Bank. GDP per capita (current US$). Available at: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD. Accessed 24 Aug 2014.
Kowada A. Cost effectiveness of interferon-γ release assay for TB screening of HIV positive pregnant women in low TB incidence countries. J Infect. 2014;68:32–42.
Maheswaran H, Barton P. Intensive case finding and isoniazid preventative therapy in HIV infected individuals in Africa: economic model and value of information analysis. PLoS One. 2012;7:e30457.
Burgos JL, Kahn JG, Strathdee SA, Valencia-Mendoza A, Bautista-Arredondo S, Laniado-Laborin R, et al. Targeted screening and treatment for latent tuberculosis infection using QuantiFERON-TB Gold is cost-effective in Mexico. Int J Tuberc Lung Dis. 2009;13:962–8.
Linas BP, Wong AY, Freedberg KA, Horsburgh CR Jr. Priorities for screening and treatment of latent tuberculosis infection in the United States. Am J Respir Crit Care Med. 2011;184:590–601.
LoBue PA, Enarson DA, Thoen TC. Tuberculosis in humans and its epidemiology, diagnosis and treatment in the United States. Int J Tuberc Lung Dis. 2010;14:1226–32.
Broekmans JF, Migliori GB, Rieder HL, et al. European framework for tuberculosis control and elimination in countries with low incidence. Recommendations of a Working Group of the World Health organization (WHO), International Union against Tuberculosis and Lung Disease (IUATLD) and Royal Netherlands Tuberculosis Association (KNCV) Working Group. Eur Respir J. 2002;19:765–75.
European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe 2012. Stockholm: European Centre for Disease Prevention and Control; 2012.
Porco TC, Lewis B, Marseille E, Grinsdale J, Flood JM, Royce SE. Cost-effectiveness of tuberculosis evaluation and treatment of newly-arrived immigrants. BMC Public Health. 2006;6:157.
Yuan L, Richardson E, Kendall PR. Evaluation of a tuberculosis screening program for high-risk students in Toronto schools. CMAJ. 1995;153:925–32.
Schwartzman K, Menzies D. Tuberculosis screening of immigrants to low-prevalence countries: a cost-effectiveness analysis. Am J Respir Crit Care Med. 2000;161(3 Pt 1):780–9.
Oxlade O, Schwartzman K, Menzies D. Interferon-gamma release assays and TB screening in high-income countries: a cost-effectiveness analysis. Int J Tuberc Lung Dis. 2007;11:16–26.
Pareek M, Watson JP, Ormerod LP, et al. Screening of immigrants in the UK for imported latent tuberculosis: a multicentre cohort study and cost-effectiveness analysis. Lancet Infect Dis. 2011;11:435–44.
Baker MA, Lin HH, Chang HY, Murray MB. The risk of tuberculosis disease among persons with diabetes mellitus: a prospective cohort study. Clin Infect Dis. 2012;54:818–25.
Corris V, Unwin N, Critchley J. Quantifying the association between tuberculosis and diabetes in the US: a case–control analysis. Chronic Illn. 2012;8:121–34.
Young F, Wotton CJ, Critchley JA, Unwin NC, Goldacre MJ. Increased risk of tuberculosis disease in people with diabetes mellitus: record-linkage study in a UK population. J Epidemiol Community Health. 2012;66:519–23.
Stevenson CR, Forouhi NG, Roglic G, Williams BG, Lauer JA, Dye C, et al. Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health. 2007;7:234.
Walker C, Unwin N. Estimates of the impact of diabetes on the incidence of pulmonary tuberculosis in different ethnic groups in England. Thorax. 2010;65:578–81.
Bumbacea D, Arend SM, Eyuboglu F, Fishman JA, Goletti D, Ison MG, et al. The risk of tuberculosis in transplant candidates and recipients: a TBNET consensus statement. Eur Respir J. 2012;40:990–1013.
Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infections diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004;38:1261–5.
Kowada A. Cost effectiveness of interferon-gamma release assay for tuberculosis screening of rheumatoid arthritis patients prior to initiation of tumor necrosis factor-α antagonist therapy. Mol Diagn Ther. 2010;14:367–73.
Swaminath A, Bhadelia N, Wang YC. Cost-effectiveness of QuantiFERON testing before initiation of biological therapy in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2444–9.
van der Have M, Oldenburg B, Fidder HH, Belderbos TD, Siersema PD, van Oijen MG. Optimizing screening for tuberculosis and hepatitis B prior to starting tumor necrosis factor-α inhibitors in Crohn’s disease. Dig Dis Sci. 2014;59:554–63.
Laskin BL, Goebel J, Starke JR, Schauer DP, Eckman MH. Cost-effectiveness of latent tuberculosis screening before steroid therapy for idiopathic nephrotic syndrome in children. Am J Kidney Dis. 2013;61:22–32.
Froelich H, Ackerson LM, Morozumi PA. Targeted testing of children for tuberculosis: validation of a risk assessment questionnaire. Pediatrics. 2001;107(4):E54.
Pradhan RP, Katz LA, Nidus BD, Matalon R, Eisinger RP. Tuberculosis in dialyzid patients. JAMA. 1974;229:798–800.
Kowada A. Cost effectiveness of the interferon-γ release assay for tuberculosis screening of hemodialysis patients. Nephrol Dial Transplant. 2013;28:682–8.
Jensen PA, Lambert LA, Lademarco MF, Ridzon R, Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1–141.
Nettleman MD, Geerdes H, Roy MC. The cost-effectiveness of preventing tuberculosis in physicians using tuberculin skin testing or a hypothetical vaccine. Arch Intern Med. 1997;157:1121–7.
Salpeter SR, Salpeter EE. Screening and treatment of latent tuberculosis among healthcare workers at low, moderate, and high risk for tuberculosis exposure: a cost-effectiveness analysis. Infect Control Hosp Epidemiol. 2004;25:1056–61.
de Perio MA, Tsevat J, Roselle GA, Kralovic SM, Eckman MH. Cost-effectiveness of interferon gamma release assays vs tuberculin skin tests in health care workers. Arch Intern Med. 2009;169:179–87.
del Campo MT, Fouad H, Solís-Bravo MM, Sánchez-Uriz MA, Mahíllo-Fernández I, Esteban J. Cost-effectiveness of different screening strategies (single or dual) for the diagnosis of tuberculosis infection in healthcare workers. Infect Control Hosp Epidemiol. 2012;33:1226–34.
Eralp MN, Scholtes S, Martell G, Winter R, Exley AR. Screening of healthcare workers for tuberculosis: development and validation of a new health economic model to inform practice. BMJ Open. 2012;2(2):e000630.
Kowada A. Cost-effectiveness of interferon-gamma release assay for entry tuberculosis screening in prisons. Epidemiol Infect. 2013;141:2224–34.
Churchyard GJ, Fielding KL, Lewis JJ, et al. A trial of mass isoniazid preventive therapy for tuberculosis control. N Engl J Med. 2014;370:301–10.
Rangaka MX, Wilkinson RJ, Boulle A, Glynn JR, Fielding K, van Cutsem G, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet. 2014;384:682–90.
Samandari T, Agizew TB, Nyirenda S, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:1588–98.
Dorman SE, Belknap R, Graviss EA, Reves R, Schluger N, Weinfurter P, et al. Tuberculosis epidemiologic studies consortium. Interferon-γ release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med. 2014;189:77–87.
Johi M, Monson TP, Johi A, Woods GL. IFN-γ release assay conversions and reversions. Challenges with serial testing in US health care workers. Ann Am Thorac Soc. 2014;11:296–302.
Zellweger JP, Rieder HL. Serial screening for latent tuberculosis infection in healthcare workers in low-risk settings. Am J Respir Crit Care Med. 2014;189:3–4.
Pai M, Banaei N. Occupational screening of health care workers for tuberculosis infection: tuberculin skin testing or interferon-γ release assays? Occup Med (Lond). 2013;63:458–60.
Lambert LA, Pratt RH, Armstrong LR, Haddad MB. Tuberculosis among healthcare workers, United States, 1995–2007. Infect Control Hosp Epidemiol. 2012;33:1126–32.
Schluger NW, Burzynski J. Variability in interferon gamma release assay results and screening for tuberculosis. A way forward? Ann Am Thorac Soc. 2014;11:1256–7.
Young F, Wotton CJ, Critchley JA, Unwin NC, Goldacre MJ. Increased risk of tuberculosis disease in people with diabetes mellitus: record-linkage study in a UK population. J Epidemiol Community Health. 2012;66:519–23.
DaveManuel.com. Inflation calculator. Available at: http://www.davemanuel.com/inflation-calculator.php. Accessed 24 Dec 2014.
McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics. 2008;26:733–44.
Stop TB Partnership. The global plan to stop TB 2011–2015. Available at: http://www.stoptb.org/assets/documents/global/plan/TB_GlobalPlanToStopTB2011-2015.pdf. Accessed 24 Dec 2014.
Acknowledgments and Funding Disclosures
The authors declare no support from any organization for the submitted work.
In the previous 5 years, Roland Diel has received a fee and/or travel reimbursement for speaking at symposia supported by Cellestis Ltd, Oxford Immunotec, Inc., and Pharmore Ltd.
Niklas Lampenius and Albert Nienhaus declare no financial relationship with any organization that could have an interest in the submitted work.
Roland Diel planned and managed the work, analyzed and interpreted the data, and produced the first draft of the manuscript. Niklas Lampenius and Albert Nienhaus reviewed and revised the manuscript, including essential amendments. Roland Diel finalized the paper, and Niklas Lampenius and Albert Nienhaus approved the final submitted version. Roland Diel acts as the overall guarantor of the manuscript content.
Author information
Authors and Affiliations
Corresponding author
Appendix: Full electronic search strategies
Appendix: Full electronic search strategies
MEDLINE
(cost effectiveness OR cost-effectiveness)
AND
(tuberculosis OR TB)
[All fields]
NHS EED (National Health Service Economic Evaluation Database)
(’tuberculosis’ OR ‘TB’ OR ‘TB disease’)
AND
(‘cost-effectiveness’ OR ‘cost effectiveness’)
[TI FROM 1960 TO 2014]
CEA Registry
‘tuberculosis’ [Fill search contents]
Rights and permissions
About this article
Cite this article
Diel, R., Lampenius, N. & Nienhaus, A. Cost Effectiveness of Preventive Treatment for Tuberculosis in Special High-Risk Populations. PharmacoEconomics 33, 783–809 (2015). https://doi.org/10.1007/s40273-015-0267-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-015-0267-x