Abstract
Background
Antipsychotic medication is a commonly prescribed drug class in individuals with autism spectrum disorder (ASD). However, the safety of these agents has not been fully assessed.
Objective
Our objective was to investigate the safety and tolerability profile of antipsychotics in individuals with ASD.
Methods
The Cochrane Library, MEDLINE, Embase and PsycINFO databases were searched up to January 2018. We included studies that reported adverse events (AEs) in participants with ASD taking first- or second-generation antipsychotic medication. The studies included in the analysis were randomized controlled trials (RCTs) and observational studies that were comparative or noncomparative and published as full text in the English language. The primary outcome of this review was AEs of any severity reported with antipsychotic use at any dose. Meta-analysis was performed on studies with child and adolescent participants to estimate the pooled prevalence of the overall AEs and the relative risk (RR) of AEs associated with antipsychotic use using a random-effects model. The Cochrane Collaboration tool and the modified Newcastle–Ottawa Scale (NOS) were used to assess the risk of bias of the included RCTs and observational studies, respectively.
Results
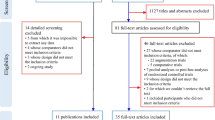
In total, 54 citations fulfilled the inclusion criteria, of which 40 were RCTs and 14 were observational studies; eight RCTs were included in the meta-analysis to estimate the RR of AEs associated with antipsychotic use and seven observational studies were included to estimate the pooled prevalence of AEs. The RR of AEs with antipsychotic treatment was 22% higher than with placebo (RR 1.22; 95% confidence interval [CI] 1.11–1.34; I2 = 30.6%; p = 0.184). The estimated pooled prevalence of AEs was 50.5% (95% CI 33–67). The most commonly reported AEs were increased appetite and weight gain, which were associated with discontinuation in many participants.
Conclusion
Antipsychotic-related AEs were common among patients with ASD. Further studies to investigate the implications of antipsychotic-related AEs on health and medication adherence are warranted. PROSPERO registration number: (CRD42018083632)




Similar content being viewed by others
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub; 2013.
Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T, Scott JG. The epidemiology and global burden of autism spectrum disorders. Psychol Med. 2015;45(3):601–13. https://doi.org/10.1017/s003329171400172x.
Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ. 2018;67(6):1.
Investigators Autism Developmental Disabilities Monitoring Network Surveillance Year Principal. Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2010. Morb Mortal Wkl Rep Surveill Summ. 2014;63(2):1–21.
Watkins EE, Zimmermann ZJ, Poling A. The gender of participants in published research involving people with autism spectrum disorders. Res Autism Spectr Disord. 2014;8(2):143–6.
Buescher AV, Cidav Z, Knapp M, Mandell DS. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatr. 2014;168(8):721–8. https://doi.org/10.1001/jamapediatrics.2014.210.
Posey DJ, McDougle CJ. Pharmacotherapeutic management of autism. Expert Opin Pharmacother. 2001;2(4):587–600. https://doi.org/10.1517/14656566.2.4.587.
National Collaborating Centre for Mental Health. Autism recognition, referral, diagnosis and management of adults on the autism spectruM. Leicester: The British Psychological Society and The Royal College of Psychiatrists; 2012.
Murray ML, Hsia Y, Glaser K, Simonoff E, Murphy DG, Asherson PJ, et al. Pharmacological treatments prescribed to people with autism spectrum disorder (ASD) in primary health care. Psychopharmacology. 2014;231(6):1011–21. https://doi.org/10.1007/s00213-013-3140-7.
Hsia Y, Wong AY, Murphy DG, Simonoff E, Buitelaar JK, Wong IC. Psychopharmacological prescriptions for people with autism spectrum disorder (ASD): a multinational study. Psychopharmacology. 2014;231(6):999–1009.
Wong A, Hsia Y, Chan EW, Murphy DG, Simonoff E, Buitelaar JK, et al. The variation of psychopharmacological prescription rates for people with autism spectrum disorder (ASD) in 30 countries. Autism Res. 2014;7(5):543–54.
Shea S, Turgay A, Carroll A, Schulz M, Orlik H, Smith I, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114(5):e634–41. https://doi.org/10.1542/peds.2003-0264-F.
Owen R, Sikich L, Marcus RN, Corey-Lisle P, Manos G, McQuade RD, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009;124(6):1533–40. https://doi.org/10.1542/peds.2008-3782.
Ichikawa H, Mikami K, Okada T, Yamashita Y, Ishizaki Y, Tomoda A, et al. Aripiprazole in the treatment of irritability in children and adolescents with autism spectrum disorder in Japan: a randomized, double-blind, placebo-controlled study. Child Psychiatry Hum Dev. 2017;48(5):796–806.
Loebel A, Brams M, Goldman R, Silva R, Hernandez D, Deng L, et al. Lurasidone for the treatment of irritability associated with autistic disorder. J Autism Dev Disord. 2016. https://doi.org/10.1007/s10803-015-2628-x.
Stigler K, Mullett J, Erickson C, Posey D, McDougle C. Paliperidone for irritability in adolescents and young adults with autistic disorder. Psychopharmacology. 2012. https://doi.org/10.1007/s00213-012-2711-3.
Pandina G, Bossie C, Youssef E, Zhu Y, Dunbar F. Risperidone improves behavioral symptoms in children with autism in a randomized, double-blind, placebo-controlled trial. J Autism Dev Disord. 2007. https://doi.org/10.1007/s10803-006-0234-7.
Hollander E, Wasserman S, Swanson E, Chaplin W, Schapiro M, Zagursky K, et al. A double-blind placebo-controlled pilot study of olanzapine in childhood/adolescent pervasive developmental disorder. J Child Adolesc Psychopharmacol. 2006. https://doi.org/10.1089/cap.2006.16.541.
Almandil NB, Liu Y, Murray ML, Besag FM, Aitchison KJ, Wong IC. Weight gain and other metabolic adverse effects associated with atypical antipsychotic treatment of children and adolescents: a systematic review and meta-analysis. Paediatr Drugs. 2013;15(2):139–50. https://doi.org/10.1007/s40272-013-0016-6.
Almandil NB, Wong IC. Review on the current use of antipsychotic drugs in children and adolescents. Arch Dis Child Educ Pract Edn. 2011;96(5):192–6. https://doi.org/10.1136/archdischild-2011-300054.
Fleischhaker C, Heiser P, Hennighausen K, Herpertz-Dahlmann B, Holtkamp K, Mehler-Wex C, et al. Clinical drug monitoring in child and adolescent psychiatry: side effects of atypical neuroleptics. J Child Adolesc Psychopharmacol. 2006;16(3):308–16. https://doi.org/10.1089/cap.2006.16.308.
Rani FA, Byrne P, Cranswick N, Murray ML, Wong IC. Mortality in children and adolescents prescribed antipsychotic medication: a retrospective cohort study using the UK general practice research database. Drug Saf. 2011;34(9):773–81. https://doi.org/10.2165/11591120-000000000-00000.
Sheehan R, Horsfall L, Strydom A, Osborn D, Walters K, Hassiotis A. Movement side effects of antipsychotic drugs in adults with and without intellectual disability: UK population-based cohort study. BMJ Open. 2017;7(8):e017406. https://doi.org/10.1136/bmjopen-2017-017406.
Star K, Iessa N, Almandil NB, Wilton L, Curran S, Edwards IR, et al. Rhabdomyolysis reported for children and adolescents treated with antipsychotic medicines: a case series analysis. J Child Adolesc Psychopharmacol. 2012;22(6):440–51. https://doi.org/10.1089/cap.2011.0134.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. Cochrane Collab. 2011;5:83–4.
Cooper H, Hedges LV, Valentine JC. Evaluating coding decisions. In: The handbook of research synthesis and meta-analysis. Russell Sage Foundation; 2009. p. 187–201.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Kroon FP, van der Burg LR, Ramiro S, Landewé RB, Buchbinder R, Falzon L, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015. https://doi.org/10.1002/14651858.cd010952.pub2.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ Br Med J. 2003;327(7414):557.
Beherec L, Lambrey S, Quilici G, Rosier A, Falissard B, Guillin O. Retrospective review of clozapine in the treatment of patients with autism spectrum disorder and severe disruptive behaviors. J Clin Psychopharmacol. 2011;31(3):341–4.
Wink LK, Early M, Schaefer T, Pottenger A, Horn P, McDougle CJ, et al. Body mass index change in autism spectrum disorders: comparison of treatment with risperidone and aripiprazole. J Child Adolesc Psychopharmacol. 2014;24(2):78–82.
Wink LK, Pedapati EV, Horn PS, McDougle CJ, Erickson CA. Multiple antipsychotic medication use in autism spectrum disorder. J Child Adolesc Psychopharmacol. 2017;27(1):91–4.
Nagaraj R, Singhi P, Malhi P. Risperidone in children with autism: randomized, placebo-controlled, double-blind study. J Child Neurol. 2006;21(6):450–5.
Owen R, Sikich L, Marcus R, Corey-Lisle P, Manos G, McQuade R, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009. https://doi.org/10.1542/peds.2008-3782.
Kent J, Kushner S, Ning X, Karcher K, Ness S, Aman M, et al. Risperidone dosing in children and adolescents with autistic disorder: a double-blind, placebo-controlled study. J Autism Dev Disord. 2013. https://doi.org/10.1007/s10803-012-1723-5.
Findling RL, Maxwell K, Wiznitzer M. An open clinical trial of risperidone monotherapy in young children with autistic disorder. Psychopharmacol Bull. 1997;33(1):155–9.
McDougle CJ, Holmes JP, Bronson MR, Anderson GM, Volkmar FR, Price LH, et al. Risperidone treatment of children and adolescents with pervasive developmental disorders: a prospective, open-label study. J Am Acad Child Adolesc Psychiatry. 1997;36(5):685–93.
Nicolson R, Awad G, Sloman L. An open trial of risperidone in young autistic children. J Am Acad Child Adolesc Psychiatry. 1998;37(4):372–6.
Masi G, Cosenza A, Mucci M. Prolactin levels in young children with pervasive developmental disorders during risperidone treatment. J Child Adolesc Psychopharmacol. 2001;11(4):389–94.
Masi G, Cosenza A, Mucci M, Brovedani P. Open trial of risperidone in 24 young children with pervasive developmental disorders. J Am Acad Child Adolesc Psychiatry. 2001;40(10):1206–14.
Masi G, Cosenza A, Mucci M, De Vito G. Risperidone monotherapy in preschool children with pervasive developmental disorders. J Child Neurol. 2001;16(6):395–400.
Kemner C, Willemsen-Swinkels SH, de Jonge M, Tuynman-Qua H, van Engeland H. Open-label study of olanzapine in children with pervasive developmental disorder. J Clin Psychopharmacol. 2002;22(5):455–60.
Malone RP, Maislin G, Choudhury MS, Gifford C, Delaney MA. Risperidone treatment in children and adolescents with autism: short- and long-term safety and effectiveness. J Am Acad Child Adolesc Psychiatry. 2002;41(2):140–7.
Gagliano A, Germano E, Pustorino G, Impallomeni C, D’Arrigo C, Calamoneri F, et al. Risperidone treatment of children with autistic disorder: effectiveness, tolerability, and pharmacokinetic implications. J Child Adolesc Psychopharmacol. 2004;14(1):39–47.
Aman MG, Arnold LE, Lindsay R, Nash P, Hollway J, McCracken JT, et al. Risperidone treatment of autistic disorder: longer-term benefits and blinded discontinuation after 6 months. Am J Psychiatry. 2005;162(7):1361–9.
Troost PW, Lahuis BE, Steenhuis M-P, Ketelaars CE, Buitelaar JK, Engeland HV, et al. Long-term effects of risperidone in children with autism spectrum disorders: a placebo discontinuation study. J Am Acad Child Adolesc Psychiatry. 2005;44(11):1137–44.
Malone RP, Delaney MA, Hyman SB, Cater JR. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17(6):779–90.
Troost PW, Lahuis BE, Hermans MH, Buitelaar JK, Van Engeland H, Scahill L, et al. Prolactin release in children treated with risperidone: impact and role of CYP2D6 metabolism. J Clin Psychopharmacol. 2007;27(1):52–7.
Capone GT, Goyal P, Grados M, Smith B, Kammann H. Risperidone use in children with Down syndrome, severe intellectual disability, and comorbid autistic spectrum disorders: a naturalistic study. J Dev Behav Pediatr. 2008;29(2):106–16.
Gencer O, Emiroglu F, Miral S, Baykara B, Baykara A, Dirik E. Comparison of long-term efficacy and safety of risperidone and haloperidol in children and adolescents with autistic disorder: an open label maintenance study. Eur Child Adolesc Psychiatry. 2008;17(4):217–25.
Stigler KA, Diener JT, Kohn AE, Li L, Erickson CA, Posey DJ, et al. Aripiprazole in pervasive developmental disorder not otherwise specified and asperger’s disorder: a 14-week, prospective, open-label study. J Child Adolesc Psychopharmacol. 2009;19(3):265–74.
Hellings JA, Cardona AM, Schroeder SR. Long-term safety and adverse events of risperidone in children, adolescents, and adults with pervasive developmental disorders. J Ment Health Res Intellect Disabil. 2010;3(3):132–44.
Marcus RN, Owen R, Manos G, Mankoski R, Kamen L, McQuade RD, et al. Safety and tolerability of aripiprazole for irritability in pediatric patients with autistic disorder: a 52-week, open-label, multicenter study. J Clin Psychiatry. 2011;72(9):1270–6.
Kent J, Hough D, Singh J, Karcher K, Pandina G. An open-label extension study of the safety and efficacy of risperidone in children and adolescents with autistic disorder. J Child Adolesc Psychopharmacol. 2013. https://doi.org/10.1089/cap.2012.0058.
Ichikawa H, Hiratani M, Yasuhara A, Tsujii N, Oshimo T, Ono H, et al. An open-label extension long-term study of the safety and efficacy of aripiprazole for irritability in children and adolescents with autistic disorder in Japan. Psychiatry Clin Neurosci. 2017;23:23.
Nikvarz N, Alaghband-Rad J, Tehrani-Doost M, Alimadadi A, Ghaeli P. Comparing efficacy and side effects of memantine vs. risperidone in the treatment of autistic disorder. Pharmacopsychiatry. 2017;50(1):19–25.
Remington G, Sloman L, Konstantareas M, Parker K, Gow R. Clomipramine versus haloperidol in the treatment of autistic disorder: a double-blind, placebo-controlled, crossover study. J Clin Psychopharmacol. 2001;21:440–4.
McCracken J, McGough J, Shah B, Cronin P, Hong D, Aman M, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002. https://doi.org/10.1056/nejmoa013171.
Anderson GM, Scahill L, McCracken JT, McDougle CJ, Aman MG, Tierney E, et al. Effects of short- and long-term risperidone treatment on prolactin levels in children with autism. Biol Psychiatry. 2007;61(4):545–50.
Marcus R, Owen R, Kamen L, Manos G, McQuade R, Carson W, et al. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry. 2009. https://doi.org/10.1097/chi.0b013e3181b76658.
Findling RL, Mankoski R, Timko K, Lears K, McCartney T, McQuade RD, et al. A randomized controlled trial investigating the safety and efficacy of aripiprazole in the long-term maintenance treatment of pediatric patients with irritability associated with autistic disorder. J Clin Psychiatry. 2014;75(1):22–30.
Ghanizadeh A, Sahraeizadeh A, Berk M. A head-to-head comparison of aripiprazole and risperidone for safety and treating autistic disorders, a randomized double blind clinical trial. Child Psychiatry Hum Dev. 2014;45(2):185–92.
Scahill L, Jeon S, Boorin S, McDougle C, Aman M, Dziura J, et al. Weight gain and metabolic consequences of risperidone in young children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2016. https://doi.org/10.1016/j.jaac.2016.02.016.
Vo L, Snyder C, McCracken C, McDougle C, McCracken J, Aman M, et al. No apparent cardiac conduction effects of acute treatment with risperidone in children with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2016. https://doi.org/10.1089/cap.2016.0090.
Luby J, Mrakotsky C, Stalets M, Belden A, Heffelfinger A, Williams M, et al. Risperidone in preschool children with autistic spectrum disorders: an investigation of safety and efficacy. J Child Adolesc Psychopharmacol. 2006. https://doi.org/10.1089/cap.2006.16.575.
Masi G, Cosenza A, Millepiedi S, Muratori F, Pari C, Salvadori F. Aripiprazole monotherapy in children and young adolescents with pervasive developmental disorders: a retrospective study. CNS Drugs. 2009;23(6):511–21.
Aman M, Rettiganti M, Nagaraja HN, Hollway JA, McCracken J, McDougle CJ, et al. Tolerability, safety, and benefits of risperidone in children and adolescents with autism: 21-month follow-up after 8-week placebo-controlled trial. J Child Adolesc Psychopharmacol. 2015;25(6):482–93.
Hellings JA, Jadhav M, Jain S, Jadhav S, Genovese A. Low dose loxapine: neuromotor side effects and tolerability in autism spectrum disorders. J Child Adolesc Psychopharmacol. 2015;25(8):618–24.
Hongkaew Y, Ngamsamut N, Puangpetch A, Vanwong N, Srisawasdi P, Chamnanphon M, et al. Hyperprolactinemia in Thai children and adolescents with autism spectrum disorder treated with risperidone. Neuropsychiatr Dis Treat. 2015;11:191–6.
Ngamsamut N, Hongkaew Y, Vanwong N, Srisawasdi P, Puangpetch A, Chamkrachangpada B, et al. 9-Hydroxyrisperidone-Induced hyperprolactinaemia in thai children and adolescents with autism spectrum disorder. Basic Clin Pharmacol Toxicol. 2016;119(3):267–72.
Nuntamool N, Ngamsamut N, Vanwong N, Puangpetch A, Chamnanphon M, Hongkaew Y, et al. Pharmacogenomics and efficacy of risperidone long-term treatment in thai autistic children and adolescents. Basic Clin Pharmacol Toxicol. 2017;121(4):316–24.
Srisawasdi P, Vanwong N, Hongkaew Y, Puangpetch A, Vanavanan S, Intachak B, et al. Impact of risperidone on leptin and insulin in children and adolescents with autistic spectrum disorders. Clin Biochem. 2017;50(12):678–85.
Masi G, Cosenza A, Mucci M, Brovedani P. A 3-year naturalistic study of 53 preschool children with pervasive developmental disorders treated with risperidone. J Clin Psychiatry. 2003;64(9):1039–47.
Corson AH, Barkenbus JE, Posey DJ, Stigler KA, McDougle CJ. A retrospective analysis of quetiapine in the treatment of pervasive developmental disorders. J Clin Psychiatry. 2004;65(11):1531–6.
Boon-Yasidhi V, Jearnarongrit P, Tulayapichitchock P, Tarugsa J. Adverse effects of risperidone in children with autism spectrum disorders in a naturalistic clinical setting at siriraj hospital, Thailand. Psychiatry J Print. 2014;2014:136158.
Vanwong N, Srisawasdi P, Ngamsamut N, Nuntamool N, Puangpetch A, Chamkrachangpada B, et al. Hyperuricemia in children and adolescents with autism spectrum disorder treated with risperidone: the risk factors for metabolic adverse effects. Front Pharmacol. 2017;7:527.
McDougle CJ, Holmes JP, Carlson DC, Pelton GH, Cohen DJ, Price LH. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry. 1998;55(7):633–41.
Brylewski J, Duggan L. Antipsychotic medication for challenging behaviour in people with learning disability. Cochrane Database Syst Rev. 2004. https://doi.org/10.1002/14651858.CD000377.pub2.
Kirino E. Serum prolactin levels and sexual dysfunction in patients with schizophrenia treated with antipsychotics: comparison between aripiprazole and other atypical antipsychotics. Ann Gen Psychiatry. 2017;16(1):43. https://doi.org/10.1186/s12991-017-0166-y.
Yasui-Furukori N, Furukori H, Sugawara N, Fujii A, Kaneko S. Dose-dependent effects of adjunctive treatment with aripiprazole on hyperprolactinemia induced by risperidone in female patients with schizophrenia. J Clin Psychopharmacol. 2010;30(5):596–9.
Chen J-X, Su Y-A, Bian Q-T, Wei L-H, Zhang R-Z, Liu Y-H, et al. Adjunctive aripiprazole in the treatment of risperidone-induced hyperprolactinemia: a randomized, double-blind, placebo-controlled, dose–response study. Psychoneuroendocrinology. 2015;58:130–40.
Moher D, Pham B, Lawson M, Klassen T. The inclusion of reports of randomised trials published in languages other than English in systematic reviews. Health Technol Assess. 2003;7(41):1–90.
Galandi D, Schwarzer G, Antes G. The demise of the randomised controlled trial: bibliometric study of the German-language health care literature, 1948 to 2004. BMC Med Res Methodol. 2006;6(1):30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
IW has received educational grants for projects unrelated to the current study from pharmaceutical companies that manufacture antipsychotic medicines and received research grants from the European Commission and Hong Kong Research Grant Council for the evaluation of antipsychotic drugs use in patients. BA, ZW, PM, FB, TA and RB have no conflicts of interest that are directly relevant to the content of this article.
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alfageh, B.H., Wang, Z., Mongkhon, P. et al. Safety and Tolerability of Antipsychotic Medication in Individuals with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Pediatr Drugs 21, 153–167 (2019). https://doi.org/10.1007/s40272-019-00333-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-019-00333-x