Abstract
Objectives
The aim of this study was to compare outcomes using two preference-based measures of health status (EQ-5D-5L and OAB-5D) in patients with overactive bladder (OAB) treated with solifenacin plus mirabegron or solifenacin monotherapy in the BESIDE trial.
Methods
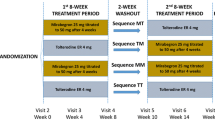
Patients with OAB who remained incontinent after 4 weeks’ treatment with solifenacin 5 mg were randomized 1:1:1 to combination treatment (solifenacin 5 mg plus mirabegron [25 mg for the first 4 weeks/50 mg for the last 8 weeks]), solifenacin 5 mg, or solifenacin 10 mg. EQ-5D-5L and OAB-q were administered at baseline, weeks 4, 8, 12, and end of treatment (EoT). OAB-5D scores were derived from OAB-q results. Responder analysis was carried out using several definitions of minimally important difference.
Results
A total of 2054 patients received one or more doses of study treatment (combination, n = 694; solifenacin 5 mg, n = 684; solifenacin 10 mg, n = 676). EQ-5D-5L Index mean score changes (from baseline to EoT) were greater with combination (0.059) than with solifenacin 5 mg (0.040) and 10 mg (0.044) monotherapy, but the differences were not statistically significant. A significantly greater improvement was observed for combination on OAB-5D (0.107 vs 0.085 for 5 mg, and 0.087 for 10 mg; p ≤ 0.01). The dimensions most improved overall were anxiety/depression, pain/discomfort, and usual activities on EQ-5D-5L, and urge, urine loss, and coping on OAB-5D. The proportion of responders was significantly greater with combination compared with monotherapy using OAB-5D only.
Conclusions
Improvements were observed in all study arms on both the EQ-5D-5L and OAB-5D, although only the OAB-5D showed a statistically significant benefit for combination versus solifenacin monotherapy. Combining generic and condition-specific preference-based health status measures allowed for assessment of dimensions that were particularly relevant to this patient population, while permitting comparison with outcomes from other studies, treatments, and populations via EQ-5D.


Similar content being viewed by others
References
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167–78.
Tubaro A. Defining overactive bladder: epidemiology and burden of disease. Urology. 2004;64(6 Suppl):2–6.
Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–36.
Nicolson P, Kopp Z, Chapple CR, Kelleher C. It’s just the worry about not being able to control it! A qualitative study of living with overactive bladder. Br J Health Psychol. 2008;13(Pt 2):343–59.
Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int. 2008;101(11):1388–95. doi:10.1111/j.1464-410X.2008.07601.x.
Tang DH, Colayco DC, Khalaf KM, Piercy J, Patel V, Globe D, et al. Impact of urinary incontinence on healthcare resource utilization, health-related quality of life and productivity in patients with overactive bladder. BJU Int. 2014;113(3):484–91. doi:10.1111/bju.12505.
Sadananda P, Drake MJ, Paton JF, Pickering AE. A functional analysis of the influence of beta3-adrenoceptors on the rat micturition cycle. J Pharmacol Exp Ther. 2013;347(2):506–15. doi:10.1124/jpet.113.207340.
Chapple CR, Kaplan SA, Mitcheson D, Klecka J, Cummings J, Drogendijk T, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a beta(3)-adrenoceptor agonist, in overactive bladder. Eur Urol. 2013;63(2):296–305. doi:10.1016/j.eururo.2012.10.048.
Khullar V, Amarenco G, Angulo JC, Cambronero J, Høye K, Milsom I, et al. Efficacy and tolerability of mirabegron, a beta(3)-adrenoceptor agonist, in patients with overactive bladder: results from a randomised European-Australian Phase 3 trial. Eur Urol. 2013;63(2):283–95. doi:10.1016/j.eururo.2012.10.016.
Nitti VW, Auerbach S, Martin N, Calhoun A, Lee M, Herschorn S. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol. 2013;189(4):1388–95. doi:10.1016/j.juro.2012.10.017.
Astellas Pharma US I. MYRBETRIQ Prescribing Information. 2012. https://www.us.astellas.com/docs/myrbetriq-full-pi.pdf. Accessed 22 June 2016.
Maman K, Aballéa S, Nazir J, Desroziers K, Neine ME, Siddiqui E, et al. Comparative efficacy and safety of medical treatments for the management of overactive bladder: a systematic literature review and mixed treatment comparison. Eur Urol. 2014;65(4):755–65. doi:10.1016/j.eururo.2013.11.010.
Sexton CC, Notte SM, Maroulis C, Dmochowski RR, Cardozo L, Subramanian D, et al. Persistence and adherence in the treatment of overactive bladder syndrome with anticholinergic therapy: a systematic review of the literature. Int J Clin Pract. 2011;65(5):567–85. doi:10.1111/j.1742-1241.2010.02626.x.
Longworth L, Yang Y, Young T, Mulhern B, Hernández Alava M, Mukuria C, et al. Use of generic and condition-specific measures of health-related quality of life in NICE decision-making: a systematic review, statistical modelling and survey. Health Technol Assess. 2014;18(8):1–224. doi:10.3310/hta18090.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. 2013. https://www.nice.org.uk/process/pmg9/. Accessed 15 Sept 2016.
Young T, Yang Y, Brazier JE, Tsuchiya A, Coyne K. The first stage of developing preference-based measures: constructing a health-state classification using Rasch analysis. Qual Life Res. 2009;18:253–65. doi:10.1007/s11136-008-9428-0.
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–36.
Drake MJ, Chapple C, Esen AA, Athanasiou S, Cambronero J, Mitcheson D, et al. Efficacy and safety of mirabegron add-on therapy to solifenacin in incontinent overactive bladder patients with an inadequate response to initial 4-week solifenacin monotherapy: a randomised double-blind multicentre Phase 3b study (BESIDE). Eur Urol. 2016;70(1):136–45. doi:10.1016/j.eururo.2016.02.030.
MacDiarmid S, Al-Shukri S, Barkin J, Fianu-Jonasson A, Grise P, Herschorn S, et al. Mirabegron as add-on treatment to solifenacin in incontinent overactive bladder patients with an inadequate response to solifenacin monotherapy: responder analyses and patient-reported outcomes from the BESIDE study. J Urol. 2016;196(3):809–18. doi:10.1016/j.juro.2016.03.174.
Devlin N, Shah K, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Office of Health Economics. Research paper 16/01. https://www.ohe.org/publications/valuing-health-related-quality-life-eq-5d-5l-value-set-england. Accessed 22 June 2016.
Feng Y, Devlin N, Shah K, Mulhern B, Van Hout B. New methods for modelling EQ-5D-5L value sets: an application to English data. Office of Health Economics. Research paper 16/02. https://www.ohe.org/publications/new-methods-modellig-eq-5d-5l-value-sets-application-english-data. Accessed 22 June 2016.
Coyne K, Revicki D, Hunt T, Corey R, Stewart W, Bentkover J, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res. 2002;11(6):563–74.
Yang Y, Brazier J, Tsuchiya A, Coyne K. Estimating a preference-based single index from the Overactive Bladder Questionnaire. Value Health. 2009;12(1):159–66. doi:10.1111/j.1524-4733.2008.00413.x.
Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–15.
Luo N, Johnson J, Coons SJ. Using instrument-defined health state transitions to estimate minimally important differences for four preference-based health-related quality of life instruments. Med Care. 2010;48(4):365–71.
Pavesi M, Devlin N, Hakimi Z, Nazir J, Herdman M, Hoyle C, et al. Understanding the effects on HR-QoL of treatment for overactive bladder: a detailed analysis of EQ-5D clinical trial data for mirabegron. J Med Econ. 2013;16(7):866–76. doi:10.3111/13696998.2013.802240.
Desroziers K, Aballéa S, Maman K, Nazir J, Odeyemi I, Hakimi Z. Estimating EQ-5D and OAB-5D health state utilities for patients with overactive bladder. Health Qual Life Outcomes. 2013;11:200. doi:10.1186/1477-7525-11-200.
Heisen M, Baeten SA, Verheggen BG, Stoelzel M, Hakimi Z, Ridder A, et al. Patient and physician preferences for oral pharmacotherapy for overactive bladder: two discrete choice experiments. Curr Med Res Opin. 2016;32:787–96. doi:10.1185/03007995.2016.1142959.
Swinburn P, Lloyd A, Ali S, Hashmi N, Newal D, Najib H. Preferences for antimuscarinic therapy for overactive bladder. BJU Int. 2011;108(6):868–73. doi:10.1111/j.1464-410X.2010.09882.x.
Kim A, Lee KS, Jung R, Na S, Kim JC, Kim HG, et al. Health related quality of life in patients with side-effects after antimuscarinic treatment for overactive bladder. Low Urin Tract Symptoms. 2016;. doi:10.1111/luts.12132.
Author information
Authors and Affiliations
Contributions
ND, JN, SM, MJD, and ZH proposed key elements of the study design and critically reviewed the draft protocol/analysis plan; MP, SM, and MJD acquired data; ND, MHe, MP, JN, SM, MJD, ES, and ZH contributed to the analysis and interpretation of the data. All authors discussed the results, provided key intellectual input and commented on the manuscript, and approved the final version for submission.
Corresponding author
Ethics declarations
Funding support
This research was funded by Astellas Pharma Europe Ltd. Medical writing support, provided by Lucy Kanan and Tyrone Daniel from Bioscript Medical, was funded by Astellas Pharma Europe Ltd.
Financial disclosures
JN, ZH, ES, and MHu are full-time employees of Astellas Pharma; MHe and ND are employees of Office of Health Economics, which was contracted by Astellas Pharma to support the conduct of this study, and members of the EuroQoL group; SM has received consultancy fees from Astellas; MJD has received speaker and advisory board fees, and research funding from Allergan, Astellas and Ferring; MP has no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Herdman, M., Nazir, J., Hakimi, Z. et al. Assessing Preference-Based Outcome Measures for Overactive Bladder: An Evaluation of Patient-Reported Outcome Data from the BESIDE Clinical Trial. Patient 10, 677–686 (2017). https://doi.org/10.1007/s40271-017-0262-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-017-0262-8