Abstract
Objective
We sought to determine the dose-response effects of extended-release (ER) dexmethylphenidate (d-MPH) and ER mixed amphetamine salts (MAS) on objective measures of sleep.
Methods
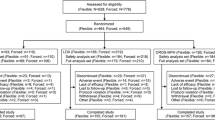
This was an 8-week, double-blind, placebo-controlled, randomized, two period, crossover study of youth with attention-deficit hyperactivity disorder (ADHD) as confirmed by the Kiddie Schedule for Affective Disorders for School-Age Children–Present and Lifetime version (K-SADS-PL). Children aged 10–17 years were recruited from clinical practice, colleague referrals, and flyers. Participants were randomized to initially receive either d-MPH or MAS. During each 4-week drug period, children received three dose levels (10, 20, and 25/30 mg) in ascending order, with placebo substituted for active medication in a randomized fashion during 1 week of the study. After 4 weeks, participants were switched to the alternative medication for another 4 weeks of treatment. The main outcome measure was sleep duration as measured by actigraphy. Children, parents, and researchers were blinded to drug, dose, and placebo status.
Results
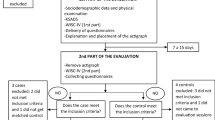
Sixty-five participants met the inclusion criteria and were enrolled in the study. Of these, 37 participants with sufficient sleep data for analysis were included. Sleep schedule measures showed a significant effect for dose on sleep start time (F(1,36) = 6.284; p < 0.05), with a significantly later sleep start time when children were receiving 20- or 30-mg doses, compared with placebo (p < 0.05). A significant dose effect was found on actual sleep duration (F(1,36) = 8.112; p < 0.05), with significantly shorter actual sleep duration for subjects receiving 30 mg compared with those receiving placebo (p < 0.05). There were no significant differences on sleep duration or sleep schedule between the two stimulant medications. The trial is complete and closed to follow-up.
Conclusions
Higher stimulant doses were associated with reduced sleep duration and later sleep start times, regardless of medication class.
Trial registration
ClinicalTrials.gov: NCT00393042.



Similar content being viewed by others
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942–8. doi:10.1176/appi.ajp.164.6.942.
Volkow ND, Tomasi D, Wang G-J, Telang F, Fowler JS, Logan J, et al. Evidence that sleep deprivation downregulates dopamine D2R in ventral striatum in the human brain. J Neurosci. 2012;32(19):6711–7. doi:10.1523/jneurosci.0045-12.2012.
Arnsten AT. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology. CNS Drugs. 2009;23(1):33–41. doi:10.2165/00023210-200923000-00005.
Safer DJ, Zito JM, Fine EM. Increased methylphenidate usage for attention deficit disorder in the 1990s. Pediatrics. 1996;98(6):1084–8.
Cox ER, Motheral BR, Henderson RR, Mager D. Geographic variation in the prevalence of stimulant medication use among children 5 to 14 years old: results from a commercially insured US sample. Pediatrics. 2003;111(2):237–43.
Dodson WW. Pharmacotherapy of adult ADHD. J Clin Psychol. 2005;61(5):589–606. doi:10.1002/jclp.20122.
Spencer TJ, Mick E, Surman CBH, Hammerness P, Doyle R, Aleardi M, et al. A randomized, single-blind, substitution study of OROS methylphenidate (Concerta) in ADHD adults receiving immediate release methylphenidate. J Atten Disord. 2011;15(4):286–94. doi:10.1177/1087054710367880.
Mitchell HA, Weinshenker D. Good night and good luck: norepinephrine in sleep pharmacology. Biochem Pharmacol. 2010;79(6):801–9. doi:10.1016/j.bcp.2009.10.004.
Lee J, Grizenko N, Bhat V, Sengupta S, Polotskaia A, Joober R. Relation between therapeutic response and side effects induced by methylphenidate as observed by parents and teachers of children with ADHD. BMC Psychiatry. 2011;11(1):70.
Stein MA, Waldman ID, Charney E, Aryal S, Sable C, Gruber R, et al. Dose effects and comparative effectiveness of extended release dexmethylphenidate and mixed amphetamine salts. J Child Adolesc Psychopharmacol. 2011;21(6):581–8. doi:10.1089/cap.2011.0018.
Gruber R, Wiebe S, Montecalvo L, Brunetti B, Amsel R, Carrier J. Impact of sleep restriction on neurobehavioral functioning of children with attention deficit hyperactivity disorder. Sleep. 2011;34(3):315–23.
Charach A, Fernandez R. Enhancing ADHD medication adherence: challenges and opportunities. Curr Psychiatry Rep. 2013;15(7):1–8.
Olfson M, Marcus S, Wan G. Stimulant dosing for children with ADHD: a medical claims analysis. J Am Acad Child Adolesc Psychiatry. 2009;48(1):51–9. doi:10.1097/CHI.0b013e31818b1c8f.
Group TMC. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1999;56(12):1073–86.
Cortese S, Holtmann M, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013;54(3):227–46. doi:10.1111/jcpp.12036.
Owen J, Sangal RB, Sutton VK, Bakken R, Allen AJ, Kelsey D. Subjective and objective measures of sleep in children with attention-deficit/hyperactivity disorder. Sleep Med. 2009;10(4):446–56.
Meltzer LJ, Walsh CM, Traylor J, Westin AM. Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep. 2012;35(1):159–66. doi:10.5665/sleep.1608.
Owens J, Sangal RB, Sutton VK, Bakken R, Allen AJ, Kelsey D. Subjective and objective measures of sleep in children with attention-deficit/hyperactivity disorder. Sleep Med. 2009;10(4):446–56. doi:10.1016/j.sleep.2008.03.013.
Golub M, Costa L, Crofton K, Frank D, Fried P, Gladen B, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of methylphenidate. Birth Defects Res Part B Dev Reprod Toxicol. 2005;74(4):300–81. doi:10.1002/bdrb.20049.
Swanson J, Volkow N. Pharmacokinetic and pharmacodynamic properties of stimulants: implications for the design of new treatments for ADHD. Behav Brain Res. 2002;130(1):73–8.
Pliszka S; AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894–921.
Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1(1):21–32.
Owens JA, Spirito A, McGuinn M. The children’s sleep habits questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–51.
Beebe DW, Lewin D, Zeller M, McCabe M, MacLeod K, Daniels SR, et al. Sleep in overweight adolescents: shorter sleep, poorer sleep quality, sleepiness, and sleep-disordered breathing. J Pediatr Psychol. 2007;32(1):69–79. doi:10.1093/jpepsy/jsj104.
DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD rating scale—IV: checklists, norms, and clinical interpretation. Guildford press: New York, NY; 1998.
Zhang S, Faries DE, Vowles M, Michelson D. ADHD rating scale IV: psychometric properties from a multinational study as clinician-administered instrument. Int J Methods Psychiatr Res. 2005;14(4):186–201. doi:10.1002/mpr.7.
Guy W. ECDEU assessment manual for psychopharmacology—revised (DHEW Pub No. ADM 76-338). Rockville: US Department of Health, Education, and Welfare. Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976: pp 218–22.
Berk M, Ng F, Dodd S, Callaly T, Campbell S, Bernardo M, et al. The validity of the CGI severity and improvement scales as measures of clinical effectiveness suitable for routine clinical use. J Eval Clin Pract. 2008;14(6):979–83. doi:10.1111/j.1365-2753.2007.00921.x.
Lauth B, Arnkelsson GB, Magnusson P, Skarpheethinsson GA, Ferrari P, Petursson H. Validity of K-SADS-PL (schedule for affective disorders and schizophrenia for school-age children-present and lifetime version) depression diagnoses in an adolescent clinical population. Nordic J Psychiatry. 2010;64(6):409–20. doi:10.3109/08039481003777484.
Jensen PS, Hinshaw SP, Swanson JM, Greenhill LL, Conners CK, Arnold LE, et al. Findings from the NIMH multimodal treatment study of ADHD (MTA): implications and applications for primary care providers. J Dev Behav Pediatr. 2001;22(1):60–73.
Jan JE, Reiter RJ, Bax MC, Ribary U, Freeman RD, Wasdell MB. Long-term sleep disturbances in children: a cause of neuronal loss. Eur J Paediatric Neurol. 2010;14(5):380–90.
Beebe DW, Rose D, Amin R. Attention, learning, and arousal of experimentally sleep-restricted adolescents in a simulated classroom. J Adoles Health. 2010;47(5):523–5.
Surman CB, Roth T. Impact of stimulant pharmacotherapy on sleep quality: post hoc analyses of 2 large, double-blind, randomized, placebo-controlled trials. J Clin Psychiatry. 2011;72(7):903–8. doi:10.4088/JCP.11m06838.
Cortese S, Faraone SV, Konofal E, Lecendreux M. Sleep in children with attention-deficit/hyperactivity disorder: meta-analysis of subjective and objective studies. J Am Acad Child Adoles Psychiatry. 2009;48(9):894–908.
Carlson GA, Kelly KL. Stimulant rebound: how common is it and what does it mean? J Child Adolesc Psychopharmacol. 2003;13(2):137–42. doi:10.1089/104454603322163853.
Acknowledgments
This study was sponsored by Novartis Pharmaceuticals, with additional support provided by the University of Illinois at Chicago Center for Clinical and Translational Science, award number UL1RR029879 from the National Center for Research Resources.
Mark A. Stein is a consultant for Novartis Pharmaceuticals, Alcobra Pharma, and Genco Pharma. He also receives research funding from Shire Plc and Pfizer.
Reut Gruber has no conflicts of interest to report.
Jose Arturo Santisteban received funding from the Mexican National Council for Science and Technology. He has no conflicts of interest to report.
Lana Bergmame has no conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santisteban, J.A., Stein, M.A., Bergmame, L. et al. Effect of Extended-Release Dexmethylphenidate and Mixed Amphetamine Salts on Sleep: A Double-Blind, Randomized, Crossover Study in Youth with Attention-Deficit Hyperactivity Disorder. CNS Drugs 28, 825–833 (2014). https://doi.org/10.1007/s40263-014-0181-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-014-0181-3