Abstract
Objective
The objective of this study was to assess the pharmacokinetic and pharmacodynamic behavior of ticagrelor administered either as crushed (in the semi-upright sitting position) or as integral (in the supine position) tablets in ST-segment elevation myocardial infarction (STEMI) patients undergoing primary percutaneous coronary intervention (PCI).
Methods
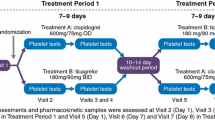
We randomized 20 patients to ticagrelor 180 mg either as 2 integral tablets administered in the supine position (standard administration) or as 2 tablets crushed and dispersed, administered in the semi-upright sitting position. Blood samples were drawn for pharmacokinetic and pharmacodynamic assessment at randomization (0 h) and at 0.5, 1, 2, and 4 h.
Results
At 1 h, ticagrelor plasma exposure and area under the plasma concentration–time curve from time zero to 1 h (AUC1) (co-primary endpoints) were higher in the crushed versus integral tablets group (median 586 vs. 70.1 ng/mL and 234 vs. 24.4 ng·h/mL, respectively), with a ratio of adjusted geometric means (95 % confidence interval [CI]) of 12.67 (2.34–68.51) [p = 0.005] and 19.28 (3.51–106.06) [p = 0.002], respectively. Time to maximum plasma concentration was shorter in the crushed versus integral tablets group (median 2 vs. 4 h), with a ratio of adjusted geometric means (95 % CI) of 0.69 (0.49–0.97) [p = 0.035]. Parallel findings were observed with AR-C124910XX (active metabolite). Platelet reactivity (VerifyNow®) at 1 h was lower with crushed versus standard administration with least squares estimates mean difference (95 % CI) of 92 (−158.4 to 26.6) P2Y12 reaction units (p = 0.009).
Conclusions
In patients with STEMI undergoing primary PCI, ticagrelor crushed tablets administered in the semi-upright sitting position seems to lead to a faster—compared with standard administration—absorption, with stronger antiplatelet activity within the first hour.
Trial registration: ClinicalTrials.gov identifier: NCT02046486.


Similar content being viewed by others
References
Alexopoulos D, Xanthopoulou I, Goudevenos J. Effects of P2Y12 receptor inhibition in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2014;113:2064–9.
Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–619.
American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions, O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, et al. ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–140.
Heestermans AA, van Werkum JW, Taubert D, Seesing TH, von Beckerath N, Hackeng CM, et al. Impaired bioavailability of clopidogrel in patients with a ST-segment elevation myocardial infarction. Thromb Res. 2008;122:776–81.
Valgimigli M, Tebaldi M, Campo G, Gambetti S, Bristot L, Monti M, et al. FABOLUS PRO Investigators. Prasugrel versus tirofiban bolus with or without short post-bolus infusion with or without concomitant prasugrel administration in patients with myocardial infarction undergoing coronary stenting: the FABOLUS PRO (Facilitation through Aggrastat By drOpping or shortening Infusion Line in patients with ST-segment elevation myocardial infarction compared to or on top of PRasugrel given at loading dOse) trial. J Am Coll Cardiol Interv. 2012;5:268–77.
Alexopoulos D, Xanthopoulou I, Gkizas V, Kassimis G, Theodoropoulos KC, Makris G, et al. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST elevation myocardial infarction. Circ Cardiovasc Interv. 2012;5:797–804.
Parodi G, Valenti R, Bellandi B, Migliorini A, Marcucci R, Comito V, et al. Comparison of prasugrel and ticagrelor loading doses in ST-segment elevation myocardial infarction patients: Rapid Activity of Platelet Inhibitor Drugs (RAPID) primary PCI study. J Am Coll Cardiol. 2013;61:1601–6.
Alexopoulos D, Gkizas V, Patsilinakos S, Xanthopoulou I, Angelidis C, Anthopoulos P, et al. Double versus standard loading dose of ticagrelor: onset of antiplatelet action in patients with STEMI undergoing primary PCI. J Am Coll Cardiol. 2013;62:940–1.
Parodi G, Bellandi B, Valenti R, Migliorini A, Marcucci R, Carrabba N, et al. Comparison of double (360 mg) ticagrelor loading dose with standard (60 mg) prasugrel loading dose in ST-elevation myocardial infarction patients: the Rapid Activity of Platelet Inhibitor Drugs (RAPID) primary PCI 2 study. Am Heart J. 2014;167:909–14.
Alexopoulos D, Makris G, Xanthopoulou I, Patsilinakos S, Deftereos S, Gkizas V, et al. Onset of antiplatelet action with high (100 mg) versus standard (60 mg) loading dose of prasugrel in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention: pharmacodynamic study. Circ Cardiovasc Interv. 2014;7:233–9.
Parodi G, Xanthopoulou I, Bellandi B, Gkizas V, Valenti R, Karanikas S, et al. Ticagrelor crushed tablets administration in STEMI patients: the MOJITO study. J Am Coll Cardiol. 2015;10(65):511–2.
Zhang X, Xiang X, Tu L, Xie X, Hou X. Esophageal motility in the supine and upright positions for liquid and solid swallows through high-resolution manometry. J Neurogastroenterol Motil. 2013;19:467–72.
Crean B, Finnie C, Crosby A. Evaluation of crushed ticagrelor tablet doses: recovery following crushing and naso-gastric tube passage ex vivo. Drugs. 2013;13:153–7.
Tantry US, Bonello L, Aradi D, Price MJ, Jeong YH, Angiolillo DJ, et al. Working Group on On-Treatment Platelet Reactivity. Consensus and update on the definition of on-treatment platelet reactivity to ADP associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62:2261–73.
Sillén H, Cook M, Davis P. Determination of ticagrelor and two metabolites in plasma samples by liquid chromatography and mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2010;878:2299–306.
Husted SE, Storey RF, Bliden K, Tantry US, Høimark L, Butler K, et al. Pharmacokinetics and pharmacodynamics of ticagrelor in patients with stable coronary artery disease: results from the ONSET–OFFSET and RESPOND studies. Clin Pharmacokinet. 2012;51:397–409.
Zafar MU, Farkouh ME, Fuster V, Chesebro JH. Crushed clopidogrel administered via nasogastric tube has faster and greater absorption than oral whole tablets. J Interv Cardiol. 2009;22:385–9.
Storey RF, Husted S, Harrington RA, Heptinstall S, Wilcox RG, Peters G, et al. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol. 2007;50:1852–6.
Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120:2577–85.
Teng R, Carlson G, Hsia J. An open-label, randomized bioavailability study with alternative methods of administration of crushed ticagrelor tablets in healthy volunteers. Int J Clin Pharmacol Ther. 2015;53:183–9.
Montalescot G, van’t Hof AW, Lapostolle F, Silvain J, Lassen JF, Bolognese L, et al. ATLANTIC Investigators. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med. 2014;371:1016–27.
Parodi G, Alexopoulos D. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med. 2014;371:2337–8.
Akers WS, Oh JJ, Oestreich JH, Ferraris S, Wethington M, Steinhubl SR. Pharmacokinetics and pharmacodynamics of a bolus and infusion of cangrelor: a direct, parenteral P2Y12 receptor antagonist. J Clin Pharmacol. 2010;50:27–35.
Bhatt DL, Stone GW, Mahaffey KW, Gibson CM, Steg PG, Hamm CW, et al. CHAMPION PHOENIX Investigators. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368:1303–13.
Parodi G, Bellandi B, Xanthopoulou I, Capranzano P, Capodanno D, Valenti R, et al. Morphine is associated with a delayed activity of oral antiplatelet agents in patients with ST-elevation acute myocardial infarction undergoing primary percutaneous coronary intervention. Circ Cardiovasc Interv. 2014;8(1):e001593. doi:10.1161/CIRCINTERVENTIONS.114.001593.
Alexopoulos D. OraL crushed and dIspersed ticagrelor 180mg compared to whole tablets of eQUal dose in STEMI patients unDergoing primary PCI: a pharmacokinetic/pharmacodynamic study (the LIQUID Study) [ClinicalTrials.gov identifier NCT02046486]. US National Institutes of Health, ClinicalTrials.gov. https://www.clinicaltrials.gov. Accessed 6 Aug 2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
This study was supported by the Research Committee of the Patras University Medical School. Dr Alexopoulos has received speaker honoraria from AstraZeneca and Advisory Board fees from AstraZeneca, Boeringer Ingelheim, Bayer, and the Medicines Company. Dr Nylander is an employee of AstraZeneca. Dr Parodi has received consulting fees from Daiichi Sankyo, Eli Lilly, AstraZeneca, Bayer, and the Medicines Company and lecture fees from Daiichi Sankyo, Eli Lilly, and AstraZeneca. Nikolaos Barampoutis, Vasileios Gkizas, Chrysoula Vogiatzi, Grigorios Tsigkas, Nikolaos Koutsogiannis, Periklis Davlouros, George Hahalis, Sven Nylander, and Ioanna Xanthopoulou have nothing to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alexopoulos, D., Barampoutis, N., Gkizas, V. et al. Crushed Versus Integral Tablets of Ticagrelor in ST-Segment Elevation Myocardial Infarction Patients: A Randomized Pharmacokinetic/Pharmacodynamic Study. Clin Pharmacokinet 55, 359–367 (2016). https://doi.org/10.1007/s40262-015-0320-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-015-0320-0