Abstract
Background
During the last decade, the implementation of biologic agents has changed the therapeutic management of severe psoriasis. Biologic agents have clinically proven efficacy, but their use is associated with a much higher cost compared with traditional treatment options. Therefore, when assessing the use of these drugs for the treatment of psoriasis, it is important to consider their cost effectiveness.
Objective
The objective of this study was to determine and compare the cost effectiveness of biologic agents with regard to the cost per patient achieving a minimally important difference (MID) in the Dermatology Life Quality Index (DLQI) and the cost per patient achieving a 75 % improvement in the Psoriasis Area Severity Index (PASI-75).
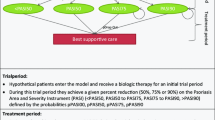
Methods
A PubMed literature search was conducted to identify studies describing the efficacy of all currently US FDA-approved biologic therapies. The cost effectiveness of each agent over a 12-week period was determined and a sensitivity analysis was performed. Based on clinical efficacy at 12 weeks, treatment paradigms were extrapolated to estimate cost-effectiveness ratios after 1 year of treatment. Pooled data on each biologic agent at different doses were compared in a one-way sensitivity analysis and in an extreme case scenario analysis.
Results
Twenty-seven studies were included in the analysis. Intravenous (IV) infliximab 3 mg/kg was the most cost-effective biologic agent with respect to both the cost per patient achieving PASI-75 and the cost per patient achieving a DLQI MID. The next most cost-effective agents in terms of cost per patient achieving PASI-75 were subcutaneous (SQ) adalimumab 40 mg administered every other week (eow) after an 80-mg loading dose, SQ adalimumab 40 mg eow, and IV infliximab 5 mg/kg. In terms of cost per patient achieving DLQI MID, IV infliximab 5 mg/kg, SQ etanercept 25 mg once weekly, SQ etanercept 50 mg once weekly, and SQ adalimumab 50 mg eow after an 80-mg loading dose were the next most cost-effective agents behind IV infliximab 3 mg/kg. For both costs per patient achieving DLQI MID and PASI-75, alefacept was the least cost-effective agent up to a 10 % level of variation at all doses except 0.025 mg/kg once weekly.
Limitations
This study was limited by the use of efficacy data from 12-week clinical trials that did not compare treatments head to head to determine relative efficacy and may not be generalizable to longer treatment periods. Additionally, the estimated cost of treatment did not take into account indirect costs or variations in costs due to insurance company price contracting.
Conclusions
Biologic treatments that were most cost effective were so in respect to both the cost per patient achieving DLQI MID and per patient achieving PASI-75. This suggests that the same agents that are effectively clearing the disease are also effective in improving the patients’ subjective assessment of dermatology-related quality of life.

Similar content being viewed by others
References
Schon MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352(18):1899–912.
Meyer N, Paul C, Feneron D, et al. Psoriasis: an epidemiological evaluation of disease burden in 590 patients. J Eur Acad Dermatol Venereol. 2010;24(9):1075–82.
Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137(3):280–4.
Singri P, West DP, Gordon KB. Biologic therapy for psoriasis: the new therapeutic frontier. Arch Dermatol. 2002;138(5):657–63.
Miller DW, Feldman SR. Cost-effectiveness of moderate-to-severe psoriasis treatment. Expert Opin Pharmacother. 2006;7(2):157–67.
Mazzotti E, Picardi A, Sampogna F, et al. Sensitivity of the Dermatology Life Quality Index to clinical change in patients with psoriasis. Br J Dermatol. 2003;149(2):318–22.
De KJ, Mombers FM, Sprangers MA, et al. The suitability of quality-of-life questionnaires for psoriasis research: a systematic literature review. Arch Dermatol. 2002;138(9):1221–7.
Puzenat E, Bronsard V, Prey S, et al. What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J Eur Acad Dermatol Venereol. 2010;24(Suppl. 2):10–6.
Nelson AA, Pearce DJ, Fleischer AB Jr, et al. Cost-effectiveness of biologic treatments for psoriasis based on subjective and objective efficacy measures assessed over a 12-week treatment period. J Am Acad Dermatol. 2008;58(1):125–35.
Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51(12):1235–41.
2010 drug topics red book: pharmacy’s fundamental reference. Montvale (NJ): Blackwell, 2008.
American Medical Association. CPT code search. https://catalog.ama-assn.org/Catalog/product/product_search.jsp?_requestid=74979. Accessed 29 Sept 2010.
Staidle JP, Dabade TS, Feldman SR. A pharmacoeconomic analysis of severe psoriasis therapy: a review of treatment choices and cost efficiency. Expert Opin Pharmacother. 2011;12(13):2041–54.
Briggs AH, Gray AM. Handling uncertainty in economic evaluations of healthcare interventions. BMJ. 1999;319(7210):635–8.
Asahina A, Nakagawa H, Etoh T, et al. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a phase II/III randomized controlled study. J Dermatol. 2010;37(4):299–310.
Saurat JH, Stingl G, Dubertret L, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158(3):558–66.
Revicki D, Willian MK, Saurat JH, et al. Impact of adalimumab treatment on health-related quality of life and other patient-reported outcomes: results from a 16-week randomized controlled trial in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2008;158(3):549–57.
Shikiar R, Heffernan M, Langley RG, et al. Adalimumab treatment is associated with improvement in health-related quality of life in psoriasis: patient-reported outcomes from a phase II randomized controlled trial. J Dermatolog Treat. 2007;18(1):25–31.
Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58(1):106–15.
Revicki DA, Willian MK, Menter A, et al. Impact of adalimumab treatment on patient-reported outcomes: results from a phase III clinical trial in patients with moderate to severe plaque psoriasis. J Dermatolog Treat. 2007;18(6):341–50.
Ellis CN, Mordin MM, Adler EY. Effects of alefacept on health-related quality of life in patients with psoriasis: results from a randomized, placebo-controlled phase II trial. Am J Clin Dermatol. 2003;4(2):131–9.
Lebwohl M, Christophers E, Langley R, et al. An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Arch Dermatol. 2003;139(6):719–27.
Ortonne JP. Clinical response to alefacept: results of a phase 3 study of intramuscular administration of alefacept in patients with chronic plaque psoriasis. J Eur Acad Dermatol Venereol. 2003;17(Suppl. 2):12–6.
Finlay AY, Salek MS, Haney J. Intramuscular alefacept improves health-related quality of life in patients with chronic plaque psoriasis. Dermatology. 2003;206(4):307–15.
Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349(21):2014–22.
van de Kerkhof PC, Segaert S, Lahfa M, et al. Once weekly administration of etanercept 50 mg is efficacious and well tolerated in patients with moderate-to-severe plaque psoriasis: a randomized controlled trial with open-label extension. Br J Dermatol. 2008;159(5):1177–85.
Reich K, Segaert S, Van de Kerkhof P, et al. Once-weekly administration of etanercept 50 mg improves patient-reported outcomes in patients with moderate-to-severe plaque psoriasis. Dermatology. 2009;219(3):239–49.
Papp KA, Tyring S, Lahfa M, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol. 2005;152(6):1304–12.
Gottlieb AB, Matheson RT, Lowe N, et al. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol. 2003;139(12):1627–32.
Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367(9504):29–35.
Menter A, Feldman SR, Weinstein GD, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56(1):31-15.
Feldman SR, Gottlieb AB, Bala M, et al. Infliximab improves health-related quality of life in the presence of comorbidities among patients with moderate-to-severe psoriasis. Br J Dermatol. 2008;159(3):704–10.
Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366(9494):1367–74.
Reich K, Nestle FO, Papp K, et al. Improvement in quality of life with infliximab induction and maintenance therapy in patients with moderate-to-severe psoriasis: a randomized controlled trial. Br J Dermatol. 2006;154(6):1161–8.
Gottlieb AB, Evans R, Li S, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004;51(4):534–42.
Feldman SR, Gordon KB, Bala M, et al. Infliximab treatment results in significant improvement in the quality of life of patients with severe psoriasis: a double-blind placebo-controlled trial. Br J Dermatol. 2005;152(5):954–60.
Langley RG, Feldman SR, Han C, et al. Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: results from a randomized, double-blind, placebo-controlled phase III trial. J Am Acad Dermatol. 2010;63(3):457–65.
Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371(9625):1675–84.
Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63(3):154–63.
Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371(9625):1665–74.
Lebwohl M, Papp K, Han C, et al. Ustekinumab improves health-related quality of life in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial. Br J Dermatol. 2010;162(1):137–46.
The National Psoriasis Foundation. Financial assistance: biologic drug manufacturer programs. http://www.psoriasis.org/accesscare/insurance/financial-assistance/biologics. Accessed Sept 13 2012.
Acknowledgments
The Center for Dermatology Research is supported by an unrestricted educational grant from Galderma Laboratories, L.P. Dr. Feldman is a consultant and speaker for Galderma, Connetics, Abbott Labs, Warner Chilcott, Centocor, Amgen, Photomedex, Genentech, BiogenIdec, and Bristol Myers Squibb. Dr. Feldman has received grants from Galderma, Connetics, Astellas, Abbott Labs, Warner Chilcott, Centocor, Amgen, Photomedex, Genentech, BiogenIdec, Coria, Pharmaderm, Ortho Pharmaceuticals, Aventis Pharmaceuticals, Roche Dermatology, 3M, Bristol Myers Squibb, Stiefel, GlaxoSmithKline, and Novartis and has received stock options from Photomedex. Ms. Ahn, Drs. Gustafson and Sandoval, and Mr. Davis have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahn, C.S., Gustafson, C.J., Sandoval, L.F. et al. Cost Effectiveness of Biologic Therapies for Plaque Psoriasis. Am J Clin Dermatol 14, 315–326 (2013). https://doi.org/10.1007/s40257-013-0030-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-013-0030-z