Abstract
Introduction
Gabapentin has potential analgesic benefits in patients with neuropathic pain, such as post-herpetic neuralgia and diabetic peripheral neuropathy neuropathic pain. However, its efficacy in women with chronic pelvic pain (CPP) remains contradictory. In the present study, we performed a systematic review and meta-analysis of randomized controlled trials (RCTs) to ascertain the efficacy of this treatment.
Methods
We systematically reviewed RCTs published in PubMed, Embase, the Cochrane Library, Web of Science, and Google Scholar databases, up to July 2021. These articles compared gabapentin with placebo or any other active treatment for CPP in women, with ‘the change in pain scores from the baseline during the first 3 and 6 months of treatment’ taken as the primary outcome. We considered reductions equivalent to 1.0 cm for primary outcomes to be clinically important.
Results
Four studies, comprising 469 participants, were included in our meta-analysis. Results revealed that the gabapentin group had significantly higher change in pain intensity scores from baseline to 3 months [weighted mean difference (WMD) − 0.61 cm; 95% confidence interval (CI) − 0.97 to − 0.25; I2 = 0%; p = 0.0009] and 6 months (WMD − 1.38 cm; 95% CI − 1.89 to − 0.88; I2 = 0%; p < 0.00001), relative to the control group. The difference of 6-month pooled result was more clinically important. Results from analysis of secondary outcomes showed that gabapentin had no beneficial efficacy during the first 3 months of treatment. Although gabapentin treatment was associated with a higher risk of dizziness and somnolence, no statistically significant differences were observed with regards to the total incidence of adverse events.
Conclusions
Overall, gabapentin could be a potential treatment option for CPP in women. However, as a pilot study, further studies are needed to explore the longer-term benefits and definite safety of this therapy in the future.
Registration Number
PROSPERO registration number CRD42021249421.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Although several clinical studies have evaluated gabapentin for treatment of chronic pelvic pain (CPP) in women, the efficacy and safety of this therapy remain controversial. |
Therefore, there is an urgent need for specific pooled effect analysis to ascertain the methods efficacy and safety. |
What was learned from the study? |
The present meta-analysis evaluated efficacy and safety of gabapentin for treatment of CPP in women. |
Results revealed that gabapentin has potential analgesic effects in this group of patients. However, gabapentin was also associated with non-severe dizziness and somnolence, compared to the placebo or standard analgesic treatment. The evidence of the therapy option gabapentin for CPP in women is clearly presented. |
In the future, studies comprising longer-term medication and follow-up are needed to validate these findings. |
Introduction
Chronic pelvic pain (CPP), which affects between 2.1% and 24% of all women worldwide, has been associated with poor quality-of-life and negative functional status [1,2,3]. Physical and psychological treatment therapies have been used to manage CPP, albeit with limited success [4,5,6,7]. Notably, pharmacotherapy remains the first-line treatment for CPP in women [8, 9], with the common analgesic gabapentin increasingly showing potential benefits for CPP patients [10].
Gabapentin, a type of γ-aminobutyrate acid analogue, plays an analgesic role mainly by affecting the nervous system [11]. Results from some previous clinical studies have demonstrated that gabapentin generates optimal analgesic effect compared to placebo during treatment of CPP in women [12,13,14]. However, these findings are not robust and conclusive due to limited sample sizes used. To address these controversies, a large-sample, multicenter, randomized, double-blind, placebo-controlled trial was performed [3]. Results revealed that pain scores were not significantly changed from baseline to 13–16 weeks in the gabapentin group [3]. Moreover, there were no significant differences in Brief Pain Inventory (BPI) pain interference scores between the gabapentin group and control group [weighted mean difference (WMD) 0.00, 95% confidence interval (CI) − 0.74 to 0.74] [3]. Overall, these studies reveal contradictory results, with regards to long- or short-term effects of gabapentin for treatment of CPP in women. What's more, although some studies have reported gabapentin-related adverse events, such as dizziness, drowsiness and mood changes, the overall safety of this therapy also remains inconclusive [3, 12,13,14].
To date, no high-quality evidence exists with regards to efficacy and safety of gabapentin for treatment of CPP in women, necessitating a comprehensive meta-analysis. In the present study, we aimed to quantify the benefits of gabapentin for treatment of CPP in women by analyzing safety and efficacy outcomes using a meta-analysis. The change in pain scores from baseline to 3 and 6 months treatment were designated as primary outcomes. Additionally, we assessed patients’ overall satisfaction after treatment, with a view of improving their integrated judgment.
Methods
Search Strategy
This systematic review and meta-analysis was performed based on the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and recommendations from the Cochrane Collaboration [15,16,17]. Additionally, the meta-analysis was registered at International Prospective Register of Systematic Reviews (PROSPERO), under registration number CRD42021249421 (https://www.crd.york.ac.uk/PROSPERO/) in May 2021. Briefly, we searched five electronic bibliographic databases, namely PubMed, Embase, the Cochrane Library, Web of Science, and Google Scholar, for articles published before April 2021. To completely include all studies that met our inclusion criteria, we performed a final search before July 2021.
Our search strategy included the following keywords: CPP, gabapentin, and randomized controlled trial (RCT). A summary of this process is shown in the Supplement Materials (eMethods 1–5). To ensure no studies were left out, we performed a manual search of the reference lists in the downloaded articles.
Eligibility Criteria and Study Selection
RCTs were included in the analysis if: (1) they randomly evaluated efficacy of gabapentin in women with CPP and compared with placebo or standard analgesic; and (2) reported at least 1 of the following outcomes: change in pain scores at multiple timepoints, the BPI pain interference scores, the proportion of patients reporting 30% or more reduction in pain scores, the overall satisfaction rate, and the rate of drug-related adverse events. On the other hand, articles were excluded if they were abstracts, conference papers, and protocols. In addition, studies with incomplete or redundant data were also excluded. There were no language restrictions during searches. What’s more, this article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Next, two reviewers (XMF and YFR) independently screened titles and abstracts of the included studies, followed by reading of the corresponding full texts. Any disagreements between them were adjudicated through a discussion with two other reviewers (YFJ and FMY).
Data Extraction
The two reviewers (HW and XY) retrieved information, namely name of the first author, year of publication, administration route, sample size, details of intervention and control, as well as outcomes, using a standardized data extraction form. Notably, authors of the articles were contacted via e-mail, for access of important data that were either unclear or missing.
Assessment of Methodologic Quality and Risk of Bias
Assessment of methodological quality was independently conducted by two reviewers (XMF and YFR) using the Cochrane Collaboration’s risk of bias tool [18, 19]. All included studies were evaluated based on six aspects, namely random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. Two other reviewers (YFJ and FMY) were called upon to discuss any inconsistencies and reach a consensus. The risk of bias in each study was categorized as high, low, or unclear, and if one or more items fell into the high-risk group, the study would be considered to have a high risk of bias. A risk-of-bias summary table for this parameter was generated using the Review Manager, version 5.3 (Cochrane Collaboration, Nordic Cochrane Centre, London, United Kingdom) as shown in Table 1 [20].
Moreover, two reviewers (XMF and XF) independent adopted the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach to evaluate evidence of bias, inconsistency, indirectness, imprecision, and publication bias for each outcome [21]. Results of these were rated high (⊕⊕⊕⊕), moderate (⊕⊕⊕◯), low (⊕⊕◯◯) , or very low (⊕◯◯◯). Any discrepancies during assessment were discussed by two other reviewers (YFJ and FMY).
Measurement of Primary and Secondary Outcomes
The primary outcome of this meta-analysis was the change in pain scores from the baseline during the first 3 and 6 months of treatment. Pain scores were defined using either the visual analogue scale (VAS) or the numeric rating scale (NRS), both of which consisted of an 11-point scale ranging from 0 to 10, representing no pain and worst imaginable pain, respectively. Secondary pain-related outcomes were defined using the BPI pain interference score, which measures pain interference in the patient's life. The score rages from 0 to 10, with higher values denoting greater interference. Another pain-related outcome was the proportion of patients reporting 30% or more reduction in pain score. Other secondary outcomes included the proportion of patients whose improvement was ‘very marked or marked’ or felt ‘satisfied or very satisfied’ about the efficacy, as well as the rate of drug-related adverse events. Notably, the overall satisfaction rate was mainly based on patients report, while the rate of drug-related adverse events was mainly defined by the total incidence as well as occurrence of common adverse events, namely dizziness, somnolence, and mood changes. Finally, in some cases, the data of 13 and 16 weeks were approximated as 3-month data for calculating the pooled effect.
Meta-analysis
Statistical analysis was performed using Review Manager 5.3. For the observation level weight, continuous and dichotomous variables were set as ‘inverse variance’ and ‘Mantel–Haenszel’ methods, respectively. For the primary outcome, we calculated WMD using the random-effects model, with a 95% CI, two-sided p values and Z-statistics. The negative mean difference implied that gabapentin treatment generated better efficacy for treatment of CPP in women, as evidenced by a significant decrease in pain scores relative to the baseline. With regard to the BPI pain interference scores, we calculated two-sided p values, Z-statistics, WMD and 95% CI. For the proportion of patients reporting 30% or more reduction in pain score, the rate of overall satisfaction, and the rate of drug-related adverse events, the two-sided p values, Z-statistics, risk ratio (RR), and 95% CI were calculated. Data followed by p < 0.05 were considered statistically significant.
Interpretation of Outcome Results
We applied the minimally clinically important difference (MCID) in pain scores to interpret results on changes in pain scores from baseline to 3 and 6 months [22]. The difference was defined to be a 1-cm change in pain score during the course of the treatment with gabapentin for CPP in women [23].
Heterogeneity, Sensitivity, and Subgroup Analysis
We applied the I2 statistics to determine heterogeneity among studies [24]. We applied the random-effects model for analysis of studies that showed significant heterogeneity (I2 > 50%), whereas the fixed-effects model was used for studies with I2 < 50%. Furthermore, we applied sensitivity analysis to further explore sources of heterogeneity in studies that had I2 > 50%.
Assessment of Publication Biases
Funnel plots, based on Egger’s test, are recommended in the assessment of publication bias for a meta-analysis comprising at least ten trials [25, 26]. In the present study, we did not generate funnel plots owing to the fact that only four trials were included.
Results
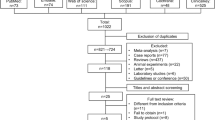
A total of 152 studies were identified across the aforementioned databases. However, only four [3, 12,13,14] met our inclusion criteria and were therefore included in the meta-analysis (Fig. 1). A total of 148 studies were excluded for various reasons. Summarily, 14 studies were excluded due to duplication, 119 studies were excluded due to unrelated intervention or comparator (n = 28), or not an RCT (n = 91) at the title and abstract screening stages. In addition, 15 articles were excluded after evaluation of full texts, of which 11 and four had no research results and missing data, respectively.
Characteristics of the Included Studies
Among the studies, two were single-center (n1 = 60, n2 = 56) (n1 = 60, n2 = 56) [13, 14], one was multicenter (n = 306) [3], while the other was a two-center (n = 47) RCTs [12]. These subjects who were included were women with a range of 30–40 years of age. With regards to pain levels, all studies analyzed women who had CPP beyond 6 months [3, 12,13,14]. Moreover, the subjects were given an oral dose of gabapentin, ranging from 300 to 3600 mg daily [3, 12,13,14]. Intervention methods in the control group applied two different methods, as evidenced by three trials that reported placebos [3, 12, 13] while the other one used standard treatment, as controls [14]. Details on characteristics of each study are shown in Table 2.
Risk of Bias
A summary of results on assessment of risk of bias across the four studies is shown in Supplement Materials (Fig. S1 and Fig. S2) [3, 12,13,14]. Overall, one study recorded a low risk of bias [3] and the other study recorded a high risk of bias [14], whereas the remaining two exhibited unclear risk of bias [12, 13]. Specifically, two trials had an unclear risk of randomization sequences [12, 14], owing to lack of detailed description of the random allocation approach. On the other hand, allocation concealment, blinding of participants, personnel and outcome assessors were not described in one trial [14], while another trial recorded unclear risk towards blinding of outcome assessors [13]. With regards to selective reporting, we found unclear risk of bias in two trials [12, 13]. For the risk of attrition bias, one exhibited ‘unclear risk of bias’ [13] and the other showed a ‘high risk of bias’ [14].
Primary Outcomes
Four trials reported ‘the change in pain scores from the baseline during the first 3 months treatment’ [3, 12,13,14]. The included subjects reported their CPP intensity change on VAS (three studies, n = 116) [12,13,14] or NRS (1 study, n = 244) [3]. In addition, three trials reported on ‘the change in pain scores from the baseline during the first 6 months treatment’ [12,13,14]. The changes in pain intensities were analyzed from baseline to 6 months (three studies, n = 99) [12,13,14] and 3 months (four studies, n = 360) [3, 12,13,14] for assessing the pooled effects.
Compared with the control group, the gabapentin group had a greater change in pain intensity scores from baseline to 3 months (WMD: − 0.61 cm; 95% CI − 0.97 to − 0.25; I2 = 0%; p = 0.0009) as well as at 6 months (WMD − 1.38 cm; 95% CI − 1.89 to − 0.88; I2 = 0%; p < 0.00001) (Fig. 2; Table 3). Changes in pain scores failed to meet the threshold for MCID (a 1-cm change at any single time point) during the 3-month period, but the changes were higher than MCID during the 6-month period. Overall, quality of evidence, for this parameter, was rated as ‘moderate’ due to a high risk of bias.
A Band plot for weighted mean difference (WMD) of the change in pain scores from baseline to 3 and 6 months between gabapentin versus control. Pooled estimates of the WMD for each time point are represented by a dark line and 95% confidence intervals are represented by the surrounding shaded region; B line chart for mean values of the change in pain scores from baseline to 3 and 6 months in two groups
Secondary Pain-Related Outcomes
The BPI Pain Interference Score
Two studies comprising 249 participants reported BPI pain interference scores [3, 12] during the first 3 months of treatment, although the pooled effect was not statistically significant (WMD − 0.01, 95% CI − 0.70 to 0.68; I2 = 0%; p = 0.97) (Table 3). For this parameter, the quality of evidence was ‘moderate’ due to a wide CI.
The Proportion of Patients Reporting 30% or More Reduction in Pain Score
Two studies, comprising 278 participants, reported this parameter [3, 13]. Notably, we could not calculate the pooled RR owing to differences in data extraction periods. One of the two studies revealed no statistically significant differences between the gabapentin (71/123) and placebo (56/121) groups during 16 weeks of treatment, whereas the other one reported a higher proportion in the gabapentin (19/20) compared to placebo (5/14) group over a 6-month period (Table 3).
Other Secondary Outcomes
The Overall Satisfaction Rate
Two studies reported the proportion of patients who felt ‘very marked or marked’ or felt ‘very satisfied or satisfied’ after treatment [3, 13]. Notably, we could not calculate the pooled RR due to the fact that the data had been collected across different timepoints. Moreover, we found no statistically significant differences between groups during both 16-week (RR = 1.49; 95% CI 0.93–2.38) and 6-months (RR = 1.31; 95% CI 0.78–2.21) treatment periods (Table 3).
The Rate of Drug-Related Adverse Events
In the included trials, there have been some drug-related adverse events in the treatment of CPP in women. Two studies [12, 14] evaluated the total incidence of adverse events (n = 87) during 6 months of treatment, while the other two [3, 13] described occurrence of common adverse events during the whole treatment period, including dizziness (n = 296), somnolence (n = 300), and mood changes (n = 290).
We found no statistically significant differences in the pooled effect, with regard to total incidence of adverse events (RR 0.50; 95% CI 0.03–7.31; I2 = 73%; p = 0.61). Quality of the evidence was considered ‘low’ due to the wide CI and high risk of bias. Furthermore, pooled results revealed no significant differences in the risk of mood changes between the groups (RR 1.23; 95% CI 0.91–1.67; I2 = 0%; p = 0.17). The quality of evidence for this parameter was ‘moderate’, due to the wide CI. However, the gabapentin group of CPP women recorded higher incidence of dizziness and somnolence, relative to the placebo. For both outcomes, the quality of the evidence was categorized as ‘moderate’ due to wide CI. The details are shown in Table 3.
Discussion
The findings of this pilot systematic review and meta-analysis provide useful insights into the longer-term potential role of gabapentin for the treatment of CPP in women. Our results revealed significant differences in pain relief outcomes between the gabapentin and placebo groups, from baseline to the first 3 and 6 months, after treatment. With regards to MCID, our results demonstrated that gabapentin is efficacious over a 6-month period but not over a 3-month treatment period. Notably, results from secondary outcomes, namely BPI pain interference scores, proportion of patients reporting 30% or more reduction in pain score, and the overall satisfaction rate, corroborated the primary results for the first 3 months. However, analysis of the proportion of patients with reduced pain scores and the overall satisfaction rates was only performed as a qualitative description. Finally, although we found no statistically significant differences in the total incidence of adverse events, additional analysis revealed that gabapentin treatment was strongly associated with a higher rate of dizziness and somnolence. Notably, gabapentin-related side effects were mild and transient, without any significant mood changes.
Collectively, our results are novel and clinically relevant. Although previous studies have described success of this therapy in the treatment of other chronic pain conditions, such as chronic neuropathic pain [27,28,29], evidence of gabapentin administration in the treatment of women with CPP remains dearth. To date, only one previous review has attempted to answer this question [10]. However, the precision and validity of findings were limited by paucity of available evidence and non-quantitative analysis, hence no intuitive answer has been obtained regarding the benefits of this therapy. We therefore designed and performed this pilot meta-analysis.
Overall, our findings revealed the potential efficacy of gabapentin for the treatment of CPP in women. Although pain relief failed to reach the MCID threshold during the first 3 months of treatment, results of pain scores over a 6-month period indicated that gabapentin requires a longer period to be embodied. Results from a previous multicenter double-blind trial indicated that gabapentin did not relieve pain in women with CPP [3]. However, it is important to note that the cycles of this trial were only performed for approximately 16 weeks. Conversely, results from another pilot trial study by Horne showed that gabapentin had a significant analgesic effect after 6 months of treatment [30]. With regards to the mechanism of action, one plausible explanation for the observed differences in time-dependent efficacy observed herein is that gabapentin exerts long-term effects on HVA ICa in medium-sized and small (IB4-) DRG neurons, which play a significant role in pain relief [31,32,33]. However, further studies using high-quality clinical designs are needed to validate gabapentin’s longer-term effects in the treatment of CPP in women.
Results of secondary outcomes corroborated those from primary outcomes, and suggested that gabapentin can generate longer-term efficacy in CPP patients. Since BPI can be used to comprehensively evaluate pain, we found two studies indicating that gabapentin does not significantly exert therapeutic effects on pain relief over a short treatment period (3 months). Generally, 30% or more pain relief is considered a good treatment outcome [22]. Our results revealed that the number of people experiencing 30% or more pain relief was higher after gabapentin treatment, relative to the placebo, over a 6-month period. However, an additional caveat is ‘the overall satisfaction rate’ used as the patient-related outcome showed that patients were not so satisfied with the efficacy of gabapentin during 16 weeks or 6 months. Therefore, gabapentin may have longer-term efficacy (≥ 6 months) in CPP patients, although further studies are warranted to validate this finding.
Our findings revealed no statistically significant differences with regards to ‘the total incidence of adverse events’ and ‘mood changes’, between the treatment and control groups, suggesting that it was a less-obvious hazard for women with CPP to receive the gabapentin treatment. However, gabapentin is closely involved in high risks of dizziness and somnolence, which are central nervous system adverse effects [34]. Therefore, patients with major neurologic conditions are advised to avoid gabapentin-based treatment [35, 36].
This review has several strengths. Firstly, we employed a comprehensive search strategy to identify and summarize our evidence. Secondly, the primary outcomes were interpreted with the MCID under a clinical context, which helped improve credibility. Thirdly, our results revealed that gabapentin has statistically significant longer-term effects (≥ 6 months) in the treatment of CPP in women, which provides a platform for future clinical studies. Finally, the low heterogeneity of the pooled effect observed herein enhanced external validity of our findings.
However, the study also had some limitations. Firstly, our study had a small sample size, which may affect the findings and conclusions of our study. Further studies using high-quality and large-scale RCTs are needed to validate our findings. Secondly, the possibility of small sample effect cannot be fully ruled out, despite that the homogeneity of data dilutes this possibility. Thirdly, it is difficult to determine the effect of age, adjuvant pain therapies, and previous pelvic surgery on efficacy of gabapentin for treatment of CPP in women using subgroup analysis owing to the lack of sufficient relevant studies. Similarly, we did not perform tests of heterogeneity and publication bias due to a limited number of relevant studies.
Conclusions
Our results demonstrate that gabapentin is a potential treatment option for CPP in women, and generates a longer-term analgesic effect. Based on results on adverse effects, we hypothesize that gabapentin treatment may not be suitable for patients with serious neurological diseases, hence they should seek alternative therapies. Further studies based on high-quality clinical trials are needed to validate our findings.
References
Polackwich AS, Shoskes DA. Chronic prostatitis/chronic pelvic pain syndrome: a review of evaluation and therapy. Prostate Cancer Prostatic Dis. 2016;19:132–8.
Franco JV, Turk T, Jung JH, et al. Non-pharmacological interventions for treating chronic prostatitis/chronic pelvic pain syndrome. Cochrane Database Syst Rev. 2018;5:CD012551.
Horne AW, Vincent K, Hewitt CA, et al. Gabapentin for chronic pelvic pain in women (GaPP2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:909–17.
Honjo H, Kamoi K, Naya Y, et al. Effects of acupuncture for chronic pelvic pain syndrome with intrapelvic venous congestion: preliminary results. Int J Urol. 2004;11:607–12.
Lachance CC, McCormack S. CADTH rapid response reports. Mindfulness training and yoga for the management of chronic non-malignant pain: a review of clinical effectiveness and cost-effectiveness. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2019.
Speer LM, Mushkbar S, Erbele T. Chronic pelvic pain in women. Am Fam Physician. 2016;93:380–7.
Meissner MH, Gibson K. Clinical outcome after treatment of pelvic congestion syndrome: sense and nonsense. Phlebology. 2015;30:73–80.
Carey ET, As-Sanie S. New developments in the pharmacotherapy of neuropathic chronic pelvic pain. Future Sci OA. 2016;2:FSO148.
Malec-Milewska M, Horosz B, Sekowska A, et al. Pharmacological treatment and regional anesthesia techniques for pain management after completion of both conservative and surgical treatment of endometriosis and pelvic adhesions in women with chronic pelvic pain as a mandated treatment strategy. Ann Agric Environ Med. 2015;22:353–6.
Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;5:CD008797.
Celikyurt IK, Mutlu O, Ulak G, Akar FY, Erden F. Gabapentin, a GABA analogue, enhances cognitive performance in mice. Neurosci Lett. 2011;492:124–8.
Lewis SC, Bhattacharya S, Wu O, et al. Gabapentin for the management of chronic pelvic pain in women (GaPP1): a pilot randomised controlled trial. PLoS ONE. 2016;11:e0153037.
AbdelHafeez MA, Reda A, Elnaggar A, El-Zeneiny H, Mokhles JM. Gabapentin for the management of chronic pelvic pain in women. Arch Gynecol Obstet. 2019;300:1271–7.
Sator-Katzenschlager SM, Scharbert G, Kress HG, et al. Chronic pelvic pain treated with gabapentin and amitriptyline: a randomized controlled pilot study. Wien Klin Wochenschr. 2005;117:761–8.
Bekele F, Mechessa DF, Sefera B. Prevalence and associated factors of the psychological impact of COVID-19 among communities, health care workers and patients in Ethiopia: a systematic review. Ann Med Surg (Lond). 2021;66:102403.
Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–84.
Yan R, Fu X, Ren YF, et al. The analgesic benefits of ketorolac to local anesthetic wound infiltration is statistically significant but clinically unimportant: a comprehensive systematic review and meta-analysis. Adv Wound Care (New Rochelle). 2021;10:583–95.
Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Koperny M, Lesniak W, Jankowski M, Bala M. The Cochrane collaboration—the role in the evolution of evidence-based medicine and development of cooperation in Poland. Przegl Epidemiol. 2016;70:508–20.
Armijo-Olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract. 2012;18:12–8.
Guyatt GH, Oxman AD, Kunz R, et al. Going from evidence to recommendations. BMJ. 2008;336:1049–51.
Fleischmann M, Vaughan B. Commentary: Statistical significance and clinical significance—a call to consider patient reported outcome measures, effect size, confidence interval and minimal clinically important difference (MCID). J Bodyw Mov Ther. 2019;23:690–4.
Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8:283–91.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74:785–94.
Liu H, Fu X, Ren YF, et al. Does inhaled methoxyflurane implement fast and efficient pain management in trauma patients? A systematic review and meta-analysis. Pain Ther. 2021;10:651–74.
Wiffen PJ, Derry S, Bell RF, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6:CD007938.
Moore RA, Wiffen PJ, Derry S, Toelle T, Rice AS. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;2014:CD007938.
Moore A, Derry S, Wiffen P. Gabapentin for chronic neuropathic pain. JAMA. 2018;319:818–9.
Horne AW, Vincent K, Cregg R, Daniels J. Is gabapentin effective for women with unexplained chronic pelvic pain? BMJ. 2017;358:j3520.
Biggs JE, Boakye PA, Ganesan N, et al. Analysis of the long-term actions of gabapentin and pregabalin in dorsal root ganglia and substantia gelatinosa. J Neurophysiol. 2014;112:2398–412.
Bowen AJ, Nowacki AS, Contrera K, et al. Short- and long-term effects of neuromodulators for unexplained chronic cough. Otolaryngol Head Neck Surg. 2018;159:508–15.
Cilio MR, Bolanos AR, Liu Z, et al. Anticonvulsant action and long-term effects of gabapentin in the immature brain. Neuropharmacology. 2001;40:139–47.
Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280:1831–6.
Rentsch CT, Morford KL, Fiellin DA, et al. Safety of gabapentin prescribed for any indication in a large clinical cohort of 571,718 US veterans with and without alcohol use disorder. Alcohol Clin Exp Res. 2020;44:1807–15.
Wilton LV, Shakir S. A postmarketing surveillance study of gabapentin as add-on therapy for 3,100 patients in England. Epilepsia. 2002;43:983–92.
Acknowledgements
Funding
This work was supported by National Administration of Traditional Chinese Medicine: 2019 Project of building evidence-based practice capacity for TCM [No. 2019XZZX-ZL006], Sichuan Provincial Key Discipline Construction Project of Traditional Chinese Medicine-Oncology of TCM [No. 2100601], and Sichuan Provincial Administration of Traditional Chinese Medicine: Special Research Project of Traditional Chinese Medicine [NO. 2021ZD003]. These projects had no role in the study design, data collection, analysis, or interpretation, or preparation of the manuscript. The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Xiu-Mei Fan: Literature search, Assessment of bias, Writing-original draft, Validation. Yi-Feng Ren: Literature search, Assessment of bias, Writing-original draft, Validation. Xi Fu: Literature search, Assessment of bias, Validation. Hao Wu: Data collection, Resources, Validation. Xin Ye: Data collection, Validation. Yi-Fang Jiang: Conceptualization, Assessment of bias, Writing-review & editing, Validation. Feng-Ming You: Conceptualization, Methodology, Assessment of bias, Writing-review & editing, Validation.
Disclosures
Xiu-Mei Fan, Yi-Feng Ren, Xi Fu, Hao Wu, Xin Ye, Yi-Fang Jiang, Feng-Ming You declare that they have no conflicts of interest related to this work to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fan, XM., Ren, YF., Fu, X. et al. Gabapentin has Longer-Term Efficacy for the Treatment of Chronic Pelvic Pain in Women: A Systematic Review and Pilot Meta-analysis. Pain Ther 10, 1673–1689 (2021). https://doi.org/10.1007/s40122-021-00330-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-021-00330-4