Abstract
Failure rates after first-line treatment of localized prostate cancer (PCa) treatment remain high. Improvements to patient selection and identification of at-risk patients are central to reducing mortality. We aimed to determine if cancer aggressiveness correlates with androgen levels in patients undergoing radical prostatectomy for localized PCa. We performed a prospective, multicenter cohort study between June 2013 and June 2016, involving men with localized PCa scheduled to undergo radical prostatectomy. Clinical and hormonal patient data (testosterone deficiency, defined by total testosterone (TT) levels < 300 ng/dL and/or bioavailable testosterone (BT) levels < 80 ng/dL) were prospectively collected, along with pathological assessment of preoperative biopsy and subsequent radical prostatectomy specimens, using predominant Gleason pattern (prdGP) 3/4 grading. Of 1343 patients analyzed, 912 (68%) had prdGP3 PCa and 431 (32%) had high-grade (prdGP4, i.e., ISUP ≥ 3) disease on prostatectomy specimens. Only moderate concordance in prdGP scores between prostate biopsies and prostatectomy specimens was found. Compared with patients with prdGP3 tumors (i.e., ISUP ≤ 2), significantly more patients with prdGP4 cancers had demonstrable hypogonadism, characterized either by BT levels (17.4% vs. 10.7%, p < 0.001) or TT levels (14.2% vs. 9.7%, p = 0.020). BT levels were also lower in patients with prdGP4 tumors compared to those with prdGP3 disease. Testosterone deficiency (defined by TT and/or BT levels) was independently associated with higher PCa aggressiveness. BT is a predictive factor for prdGP4 disease, and evaluating both TT and BT to define hypogonadism is valuable in preoperative assessment of PCa (AndroCan Trial: NCT02235142).
Similar content being viewed by others
Introduction
Radical prostatectomy (RP) is a standard first-line treatment option for localized prostate cancer (PCa), with nerve-sparing surgery restricted to preoperatively potent patients without high-grade prostatic cancer (HGPC) [1,2,3]. Less invasive treatment options (e.g., active surveillance or high-intensity focalized ultrasound (HIFU) ablation) are available but appropriate only for less aggressive cancers but with a recurrence rate that remains high at approximately 30% [4]. This may be due, in part, to the difficulty of selecting the most appropriate patients for each treatment option, which remains problematic even with the benefit of nuclear magnetic resonance imaging [5]. To improve outcomes, patient selection needs to be further refined; in this context, androgen hormonal status merits further consideration.
The impact of hypogonadism or testosterone deficiency on localized PCa remains controversial [6, 7]. Many studies have methodological limitations, e.g., small sample size or suboptimal assay protocols. Furthermore, in most studies, hypo-gonadism is based solely on total serum testosterone (TT) levels, without considering bioactive (bioavailable) testosterone (BT) which accounts for approximately 50% of TT [6]. Similarly, the complexities of the androgen pathway leading to testosterone (and allied hormonal levels) are rarely fully considered. Practical issues, such as the recommendation to perform blood collection in the morning between 7 and 11 a.m., are also often overlooked [8, 9], while radioimmunoassay (RIA) has largely been superseded by gas chromatography-mass spectrometry (GC-MS) [10, 11].
The aim of this prospective, multicenter study was to correlate aggressiveness of the PCa, as defined by the predominant Gleason pattern (prdGP) with androgen levels and behavioral/quality of life (QoL) assessments [13, 14].
Methods
Study Design and Study Population
This was a prospective cohort study, drawn from four large French metropolitan hospitals between June 2013 and June 2016, recruiting consecutive patients scheduled to undergo RP (in accordance with both the EAU and AUA recommendations for clinically localized PCa (T1-T2c)) [2, 3]. We excluded patients with previous or ongoing local treatment (e.g., radiotherapy, phototherapy, thermotherapy, HIFU) or systemic treatment likely to interfere with hormonal status (e.g., androgen receptor blockers, LHRH agonists/analogues, testosterone supplementation) or with comorbidities likely to affect gonadal status. No age or PSA level limitations were set.
The study protocol was approved by the institutional review boards of all centers. The trial was registered (NCT02235142) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients.
Assessments
Following admission for RP, demographic and anthropometric data and routine laboratory samples (including metabolic syndrome parameters) were collected on all patients. For QoL assessment, patients completed two different questionnaires; the aging male symptom (AMS) scale and the International Index of Erectile Function 5-item (IIEF-5) questionnaire [12, 13].
Assessment of Androgen Levels and PSA
Immediately prior to surgery, blood samples (30 mL) were taken between 7 and 10 a.m. following an overnight fast, as recommended in the Endocrine Society guidelines [8] and stored at − 20 °C before assay by a single laboratory. PSA, follicle-stimulating hormone (FSH), luteinizing hormone (LH), and sex hormone–binding globulin (SHBG) were measured by RIA, and TT, dihydrotestosterone (DHT), dehydroepiandrosterone (DHEA), Δ5 (androstenediol, D5), ∆4 (androstenedione, D4), estrone (E1), estradiol (E2), and DHEA sulfate (DHEA-S) were measured by GC-MS [11]. BT and free testosterone (FT) were calculated through a standardized formula using specific association constants of testosterone for SHBG and albumin. The threshold for hypogonadism was set at TT < 300 ng/dL (< 10.4 nmol/L), with a corresponding BT level set at the lower level of physiologic BT (< 80 ng/dL [< 2.8 nmol/L]).
Prostate Histological Study
Preoperative prostate core biopsies (12 from each patient) and RP specimens were processed and analyzed according to standard procedures [14]. As PCa aggressiveness is not sufficiently represented by a single Gleason score—which does not distinguish between (prognostically different) 3 + 4 and 4 + 3 scores—we used prdGP grading, with prdGP4 defining HGPC or ISUP ≥ 3, an approach proposed by Epstein et al. [15]. To ensure reporting consistency, core biopsies and RP specimens were assessed by a single pathologist at each center, then blindly centrally reviewed by a single reference uropathologist, with any differences resolved by consensus.
Data Analysis
Based on previous experience [16], we anticipated that prdGP4 would be identified in approximately one third of surgical resections. Using a margin of error of < 2.5% with 95% confidence intervals (CI), we calculated a required sample size of approximately 1350 patients; accounting for an estimated attrition rate of 20%, the recruitment target was set at 1620.
For continuous variables, data are presented as median (25th–75th percentile); for categorical variables, counts and percentages are presented. The cohort was classified into two groups according to the prdGP3 or prdGP4 grading on RP specimens. For comparisons between different levels of hypogonadism, patients were stratified into four groups: (i) “T+, B+” (TT ≥ 300 ng/dL; BT ≥ 80 ng/dL); (ii) “T+, B−” (TT ≥ 300 ng/dL; BT < 80 ng/dL); (iii) “T−, B+” (TT < 300 ng/dL; BT ≥ 80 ng/dL); (iv) “T−, B−” (TT < 300 ng/dL; BT < 80 ng/dL).
For continuous variables, differences between groups were analyzed using the non-parametric Kruskal-Wallis test, and when global p values were significant, pairwise Dunn tests were performed. The Kruskal-Wallis test was also used to compare lower grade (prdGP3) and higher grade (prdGP4) PCa. For categorical variables, Fisher’s exact test (2 × 2 or r × c) was used, and when a global significance was present, 2 × 2 Fisher’s exact test was used for pairwise comparisons. For multiple pairwise comparisons, the Bonferroni-Holms correction was used.
Multivariable logistic regression analysis was used to identify factors associated with the likelihood of upgrading from prdGP3 on biopsy to prdGP4 on RP specimens. For patients with prdGP3 on biopsy, a total of 110 logistic regressions were performed using all continuous and categorical parameter values available prior to surgery; for each regression, a random sample of 55% of cases was drawn to construct the model, with the remaining 45% used for model validation [17]. Prediction of upgrading to prdG4 on RP was at least as good as chance in 24 models, and those parameters that featured in at least two of these models were retained for the final logistic regression. Receiver operator characteristic (ROC) curve analysis was used to determine the predictive accuracy of this multivariate model.
Statistical analysis was conducted using NCSS, version 11 (NCSS Ltd., Kaysville, UT, USA) and R (version 3.3.2; The R Foundation, Vienna, Austria). A two-sided p value < 0.05 was used for statistical significance.
Results
Study Population
During the enrollment period, 2073 RPs were performed. Of these, 1611 patients were enrolled and 1343 patients were evaluable. Twenty patients (1.2%) who did not undergo RP, 147 patients (9.1%) with protocol deviations, 24 patients (1.5%) with unevaluable hormonal blood samples, and 80 patients (5.0%) who withdrew consent were excluded from the final analysis (Fig. 1).
Demographic and Clinic-Pathological Characteristics
Of the 1343 patients, 68% (n = 912) had prdGP3 (i.e., ISUP ≤ 2) tumors and 32% with prdGP4 (i.e., ISUP ≥ 3) tumors (n = 431). Characteristics of the overall cohort and stratified by prdGP grading are shown in Table 1. Compared to patients with prdGP3 tumors, patients with prdGP4 PCa were older (by approximately 1.5 years), with significantly higher PSA levels (by approximately 2.5 units), with a lower proportion of tumors at T1c stage (44% vs. 57%) and a higher proportion at clinical/nodal stages (pT ≥ 3a, pN ≥ 1). No differences in medical comorbidities and allied biochemistry were apparent, and no differences in AMS scale and IIEF-5 questionnaires were observed.
Androgen Characteristics
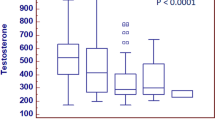
Clear differences in androgen pathway hormone levels were seen with different tumor grades (Table 1). Compared with patients with prdGP3 tumors, significantly more patients with prdGP4 cancers had demonstrable hypogonadism, characterized either by BT levels (< 80 ng/dL; 17.4% vs. 10.7%, p < 0.001) or by TT levels (< 300 ng/dL; 14.2% vs. 9.7%, p = 0.02). BT levels were also lower in those patients with prdGP4 tumors compared with those in prdGP3 patients (119 ng/dL [25th–75th percentile, 92–150 ng/dL] vs. 124 ng/dL [25th–75th percentile, 99–156 ng/dL] respectively, p = 0.008), as were FT values (and conversely substantially higher SHBG levels). Median LH and TT values did not differ significantly between prdGP3 and prdGP4 patients, with no significant difference in other steroid levels, regardless of an adrenal (i.e., DHEA, ∆5) or testicular origin (i.e., ∆4, E2, E1, or DHT).
Compared with eugonadal patients (i.e., “T+, B+”), both groups of hypogonadal patients (TT < 300 ng/dL) had significantly higher values for the main markers of the metabolic syndrome and were more likely to have cardiovascular disease, although there was no significant difference in the prevalence of diabetes (Table 2). While no significant differences in PSA values or the incidence of prdGP4 on presurgery biopsies between hypogonadal and eugonadal groups were apparent, significantly more RP specimens were graded as prdGP4 in fully hypogonadal patients (T−B−) compared to fully eugonadal (T+B+) subjects (47.5% vs. 29.9%, p = 0.004). The correlation between PSA and BT or TT was extremely low (Spearman’s coefficient of 0.018 and 0.017, respectively). The proportion of patients with prdGP4 tumors in both groups with low BT (< 80 ng/dL) was about 10% greater than in groups with higher BT, and higher tumor clinical/nodal stages were also seen in hypogonadal patients (Table 2). Significant differences in SHBG concentrations were seen for every pairwise comparison, and SHBG concentrations were higher in both TT ≥ 300 ng/dL groups. The global p values for differences in the androgen pathway hormone levels (DHEA, DHEA−S, ∆4, ∆5, TT, BT, FT, DHT, E1, E2) were all highly significant. Higher AMS sexual and IIEF-5 scores were observed in eugonadal patients compared to that in hypogonadal patients.
Multivariate logistic regression was performed on all presurgical parameters and replicated to select parameters that retained in at least 2 out of 24 models with suitable predictive value for prdGP4 on RP resection. Increases in age, PSA, triglycerides, and FSH; decreased LH and BT levels; non-Caucasian ethnicity; and coexisting cardiovascular disease each independently increase the risk of upgrading from prdGP3 on biopsy to prdGP4 on RP specimen and were included in the final model (Table 3). The corresponding integrated ROC curve for the final model is shown in Fig. 2. The model provided a predictive accuracy of 61%.
ROC curve analysis for model as a predictor of upgrading from prdGP3 on biopsy to prdGP4 on resection specimen. Model incorporates all independent predictors of prdGP4 identified in multivariate logistic regression. Model for Logit(prdG4): X = − 3.22 + 0.04 × age + 0.04 × PSA + 0.18 × triglycerides + 0.03 × FSH − 0.08 × LH − 0.11 × BT + 0.17 × non-Caucasian ethnic group + 0.69 × presence of cardiovascular disorder, with Prob(Y = grade 4) = 1/(1 + Exp(−X))
Discussion
The impact of testosterone deficiency on PCa remains controversial, with conflicting results due in part to variations in study design/methodological limitations [6]. In the present study, we applied current recommended standards of androgen evaluation, including morning androgen measurements, centralized steroid determination by GC-MS in a single laboratory [11], and a fuller evaluation of the androgen cascade, measuring not only TT but also its precursors and metabolites and in particular the biologically active form of testosterone (BT) and androgen plasma–binding globulin (SHBG).
As most circulating testosterone is unavailable to prostatic cells, being tightly bound to SHBG, BT typically only represents 50% of TT [6]. Although prostatic tissue is sensitive to changes in serum TT at low concentrations, a saturation limit exists at higher androgen levels [18]. Arguably, BT is a better indicator of circulating androgens available to target organs, and so any possible impact of hypogonadism on PCa should be evaluated using both TT and also BT levels. However, to date, few studies have suggested a correlation between low BT and high-grade PCA. Indeed, most studies do not specifically assay BT; usually, FT is calculated from a RIA measurement of TT; then, BT values are calculated based on standard formulae. The role of BT remains infrequently reported. In their pooled analysis of 18 studies recruiting 3886 men with incident PCa, Roddam and colleagues did not report BT levels [19], although more recently, Garcia-Cruz et al. reported an almost fivefold increased risk of detection of PCa on biopsy in patients with low BT [20].
In the present study, we found that BT appeared twice in a random sample of 24 models that had good predictive quality for HGPC or ISUP ≥ 3 (i.e., prdGP4) making it a candidate for the subset of variables that were to be included in such a model; in contrast, TT was not present in any of these models. Hypogonadic patients had a higher rate of prdGP4, with a lower risk in patients with physiological TT levels (Table 2). No significant differences in adrenal (i.e., DHEA, ∆5) or testicular (i.e., ∆4, E2, E1, TT, or DHT) steroid levels in patients with different tumor grades were observed. However, in prdGP4 patients, BT and FT levels were substantially and significantly lower (Table 1), a direct consequence of higher SHBG levels in patients with prdGP4 disease.
We suggest that using both TT and BT to define hypo-gonadism is a highly relevant refinement in terms of pathology, androgen cascade, and clinical parameters; testosterone deficiency should not be defined using TT in isolation. In this study, testosterone deficiency based on TT alone was found in 11.2% (14.2% in prdGP4), while adding cases based on BT increased this percentage by 8.3% (10.7% in prdGP4) leading to a total of 19.5% cases (24.9% in prdGP4); data similar to that was reported elsewhere [7]. Our data also support the growing evidence that testosterone deficiency is independently associated with higher tumor aggressiveness. Our results have implications for patient selection for nerve-sparing surgery, as EAU guidelines highlight that predominant Gleason grade 4 jeopardizes outcomes of nerve-sparing RP [1, 2].
We found weak concordance between Gleason scores of biopsy samples and full RP specimens; 21.1% of our 1045 patients with a prdGP3 biopsy had prdGP4 cancer on surgical specimens. More surprisingly, in 75 (26.8%) of the 279 patients with prdGP4 on biopsy, no prdGP4 could be detected on the surgical specimen. Although other studies report concordance ranging between 30 and 67% [21], the moderate concordance we found was despite systematic sampling (12 core biopsies) and centralized reviewing of biopsies and prostatectomy specimens by a single reference uropathologist. Discordance between biopsy and RP specimen grading is not necessarily problematic, as long as this is considered in clinical decision-making. In this context, we and others have previously reported that Gleason scores are more often under-evaluated by prostate biopsies in hypogonadal patients [16, 22], which further supports the view that hormonal status should be fully considered in PCa case assessment.
In a previous study, we found that obesity and hypo-gonadism, while not interdependent, can coexist and together may be associated with more aggressive disease [23]. Even if obesity is not the primary cause of hypogonadism, there is a large overlap between obesity and hypogonadism, and both impact PCa status. In the present study, we found that low preoperative serum TT levels, obesity, and metabolic syndrome parameters were not independently associated with aggressive features of PCa (such as extracapsular extension) nor with tumor grading and that weight, BMI, and body fat are not predictive factors of HGPC. We also found a relationship between obesity and low TT levels (but not low BT levels). This, and the fact that BT, not TT, played a role in some of the models developed to predict prdGP4 in patients with prdGP3 on biopsy, suggests a dichotomy in the role of TT and BT; namely, TT relates to adiposity and BT to PCa aggressiveness. To our knowledge, this dichotomy has not previously been suggested. One possible explanation is that BMI and metabolic syndrome parameters are only surrogate indicators of low levels of circulating androgens in PCa patients. Indeed, metabolic syndrome has been shown to result in decreased FT and BT levels in PCa, although other biological mechanisms may influence the association of metabolic disorders and aggressive PCa [24]. Higher SHBG levels were also associated with a decrease in BT, with TT levels essentially remaining normal. This may explain why obesity and diabetes can lead to hypogonadism in aging males.
The role of SHBG as a predictor of PCa extracapsular extension in men undergoing RP has previously been reported [25], although that group subsequently reported that SHBG levels are not multivariate predictors of high-risk PCa (as defined by the NCCN Practice guidelines) [26, 27], highlighting the importance of using consistent, well-defined terminology across studies evaluating PCa. Our results provide some support for predictive benefit of SHBG, with higher levels in patients with prdGP4 tumors compared to prdGP3 patients.
Finally, while we found no statistically significant differences in E1 and E2 levels between prdGP3 and prdGP4 patient groups, the absolute levels (25.6 to 26.1 pg/mL for E2), determined by GC-MS, were lower than levels (determined by RIA) reported previously by Salonia et al., where a significant association between the rate of HGPC and E2 level ≥ 50 pg/mL was seen in a small patient subset [28]. In the present study, using GC-MS, we found relatively few patients (1%) with E2 ≥ 50 pg/mL and suggest this cutoff level is not relevant for clinical practice.
Our study has strengths and limitations. The prospective multicenter aspect allowed consecutive enrolment of a com-paratively large number of patients (none of whom had received testosterone therapy or had comorbidity-associated hypo-gonadism) with data collected in a consistent manner and little if any selection bias. All RP specimens were graded by a single experienced specialist pathologist (using the most recent criteria.) Another distinguishing feature (which may help explain the discrepancy with previous study findings) was our use of a single central laboratory with analyses by GC-MS [11] and sampling according to recent recommendations [8]. Nevertheless, limitations exist; to fully assess differences influenced by any BT-TT interaction, a very large trial is required. Although total patient numbers in this study are comparatively high, less than 20% of our patients were hypogonadal, as assessed by either TT and/or BT, and so our results should be viewed in this context.
In conclusion, we found that testosterone deficiency (defined by TT and/or BT levels) was independently associated with higher PCa aggressiveness. Furthermore, BT is a key hormonal marker, and accurate assessment of BT levels is necessary when evaluating hypogonadism in patients with localized PCa. A previously undescribed dichotomy was observed, TT having a stronger link with adiposity and BT a stronger link to cancer aggressiveness. SHBG levels should also be considered in treatment decisions.
References
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N, European Association of Urology (2014) EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 65:124–137
Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, de Santis M, Fossati N, Gross T, Henry AM, Joniau S, Lam TB, Mason MD, Matveev VB, Moldovan PC, van den Bergh RCN, van den Broeck T, van der Poel HG, van der Kwast TH, Rouvière O, Schoots IG, Wiegel T, Cornford P (2017) EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 71:618–629
Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, Freedland SJ, Greene K, Klotz LH, Makarov DV, Nelson JB, Rodrigues G, Sandler HM, Taplin ME, Treadwell JR (2018) Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part II: recommended approaches and details of specific care options. J Urol 199:990–997
Dall'Era MA, Albertsen PC, Bangma C et al (2012) Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol 62:976–983
Slaoui H, Neuzillet Y, Ghoneim T, Rouanne M, Abdou A, Lugagne-Delpon PM, Scherrer A, Radulescu C, Delancourt C, Molinié V, Lebret T (2017) Gleason score within prostate abnormal areas defined by multiparametric magnetic resonance imaging did not vary according to the PIRADS score. Urol Int 99:156–161
Klap J, Schmid M, Loughlin KR (2015) The relationship between total testosterone levels and prostate cancer: a review of the continuing controversy. J Urol 193:403–413
Morgentaler A, Zitzmann M, Traish AM, Fox AW, Jones TH, Maggi M, Arver S, Aversa A, Chan JCN, Dobs AS, Hackett GI, Hellstrom WJ, Lim P, Lunenfeld B, Mskhalaya G, Schulman CC, Torres LO (2016) Fundamental concepts regarding testosterone deficiency and treatment: International Expert Consensus Resolutions. Mayo Clin Proc 91:881–896
Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM, Task Force, Endocrine Society (2010) Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 95:2536–2559
Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB (2009) The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab 94:907–913
Handelsman DJ, Wartofsky L (2013) Requirement for mass spectrometry sex steroid assays in the Journal of Clinical Endocrinology and Metabolism. J Clin Endocrinol Metab 98:3971–3973
Giton F, Trabado S, Maione L, Sarfati J, le Bouc Y, Brailly-Tabard S, Fiet J, Young J (2015) Sex steroids, precursors, and metabolite deficiencies in men with isolated hypogonadotropic hypogonadism and panhypopituitarism: a GCMS-based comparative study. J Clin Endocrinol Metab 100:E292–E296
Heinemann LA, Saad F, Zimmermann T et al (2003) The Aging Males’ Symptoms (AMS) scale: update and compilation of international versions. Health Qual Life Outcomes 1:15
Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM (1999) Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 11:319–326
Epstein JI, Allsbrook WC Jr, Amin MB et al (2005) The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol 29:1228–1242
Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, Vickers AJ, Parwani AV, Reuter VE, Fine SW, Eastham JA, Wiklund P, Han M, Reddy CA, Ciezki JP, Nyberg T, Klein EA (2016) A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur Urol 69:428–435
Pichon A, Neuzillet Y, Botto H, Raynaud JP, Radulescu C, Molinié V, Herve JM, Lebret T (2015) Preoperative low serum testosterone is associated with high-grade prostate cancer and an increased Gleason score upgrading. Prostate Cancer Prostatic Dis 18:382–387
Collins GS, Ogundimu EO, Altman DG (2016) Sample size considerations for the external validation of a multivariable prognostic model: a resampling study. Stat Med 35:214–226
Morgentaler A, Traish AM (2009) Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol 55:310–320
Endogenous H, Prostate Cancer Collaborative G, Roddam AW et al (2008) Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst 100:170–183
Garcia-Cruz E, Carrion Puig A, Garcia-Larrosa A et al (2013) Higher sex hormone-binding globulin and lower bioavailable testosterone are related to prostate cancer detection on prostate biopsy. Scand J Urol 47:282–289
D'Elia C, Cerruto MA, Cioffi A et al (2014) Upgrading and upstaging in prostate cancer: from prostate biopsy to radical prostatectomy. Mol Clin Oncol 2:1145–1149
San Francisco IF, Rojas PA, DeWolf WC et al (2014) Low free testosterone levels predict disease reclassification in men with prostate cancer undergoing active surveillance. BJU Int 114:229–235
Neuzillet Y, Raynaud JP, Lebret T, Pichon A, Radulescu C, Molinie V, Botto H (2015) Obesity and hypogonadism are associated with an increased risk of predominant Gleason 4 pattern on radical prostatectomy specimen. Horm Mol Biol Clin Investig 22:101–109
De Nunzio C, Aronson W, Freedland SJ et al (2012) The correlation between metabolic syndrome and prostatic diseases. Eur Urol 61:560–570
Salonia A, Gallina A, Briganti A, Zanni G, Suardi N, Capitanio U, Colombo R, Bertini R, Freschi M, Guazzoni G, Rigatti P, Montorsi F (2011) Sex hormone-binding globulin is a significant predictor of extracapsular extension in men undergoing radical prostatectomy. BJU Int 107:1243–1249
Salonia A, Abdollah F, Capitanio U, Suardi N, Briganti A, Gallina A, Colombo R, Ferrari M, Castagna G, Rigatti P, Montorsi F (2012) Serum sex steroids depict a nonlinear u-shaped association with high-risk prostate cancer at radical prostatectomy. Clin Cancer Res 18:3648–3657
Network NNCC (2011) Guidelines for prostate cancer, NCCN (National Comprehensive Cancer Network)
Salonia A, Gallina A, Briganti A, Suardi N, Capitanio U, Abdollah F, Bertini R, Freschi M, Rigatti P, Montorsi F (2011) Circulating estradiol, but not testosterone, is a significant predictor of high-grade prostate cancer in patients undergoing radical prostatectomy. Cancer 117:5029–5038
Acknowledgements
The authors wish to thank the subjects and healthcare staff who participated in this study and Chris Hintze (NCSS, LLC) for assistance with preliminary logistic regression model development.
Support
This study was funded by the Foch Foundation, a non-profit institution, and a grant of the French Ministry of Health/DGOS/CRC3F. Medical writing support was provided by Iain O’Neill on behalf of Newmed Medical Publishing, funded by the Foch Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neuzillet, Y., Raynaud, JP., Dreyfus, JF. et al. Aggressiveness of Localized Prostate Cancer: the Key Value of Testosterone Deficiency Evaluated by Both Total and Bioavailable Testosterone: AndroCan Study Results. HORM CANC 10, 36–44 (2019). https://doi.org/10.1007/s12672-018-0351-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-018-0351-8