Abstract
Graft-versus-host disease (GVHD) remains as the major obstacle for successful hematopoietic stem cell transplant (HSCT). Roughly half of the patients undergoing HSCT develop GVHD which requires treatment, and above 10 % of the patient may die because of it. However, GVHD presents with anti-tumor activity, called graft-versus-tumor (GVT) effect, and it carries significant anti-tumor activity, thus suppressing GVHD completely may increase the relapse of original disease. Thus, it is important to control GVHD to the appropriate level.
Similar content being viewed by others
Introduction
After the first description of stem cell infusion in patients by Thomas et al. in 1957 [1], hematopoietic stem cell transplantation (HSCT) has evolved into the treatment of choice for many hematologic malignancies and benign disorders. Increasing number of HSCT are being done every year [2]. The main benefit of allogeneic HSCT is graft vs leukemia (GVL) or graft vs tumor effect which helps in achieving cure [3]. The indications of allogeneic HSCT has expanded in the recent years especially in elderly patients owing to the advancement in reduced intensity conditioning (RIC) and in patients without HLA-matched donors due to the use of cord blood and haploidentical HSCT [4–6]. Although marked improvement has been made in supportive care, immunosuppressive therapy and DNA-based Human Leukocyte Antigen (HLA) typing, graft vs host disease (GVHD) remains a major cause of morbidity and non-relapse mortality among allogeneic HSCT recipients. It was first described by Billingham in 1966 as a syndrome where immunocompetent T cells from the donor recognize and damage the host tissue in an immunocompromised recipient. It presents with heterogeneous symptoms involving multiple organ systems including gastrointestinal tract, skin, mucosa, liver and lungs [7]. In the past clinical features occurring within 100 days after HSCT was called acute GVHD (aGVHD) and those happening after 100 days were labeled chronic GVHD (cGVHD) [8, 9]. This definition was rather unsatisfactory thus National Institute of Health (NIH) consensus criteria were developed and have been revised. The criteria have added new categories such as late-onset aGVHD (acute GVHD occurring after 100 days) and overlap syndrome which includes features of both acute and chronic GVHD to the classification, and also have introduced new definitions for organ system involvement [10–12]. The categorization of acute vs chronic GVHD is based on the combination of clinical symptoms rather than the time of onset. Depending upon a number of variables associated with patients, donors, and types of transplant, the incidence of aGVHD varies with incidence of grade II–IV GVHD at 40 % in matched related donor (MRD) transplant to 50 % in MUD transplant. The main risk factors for aGVHD include degree of HLA mismatch, age of the patient, previous all immunization of the donor and the kind of GVHD prophylaxis used. About 30–70 % of allogeneic HSCT recipients alive after 100 days will develop cGVHD. It is associated with reduced relapse rate in patients with acute leukemia, but is the leading cause of late death in patients. The incidence of acute and chronic GVHD probably will increase in the future with increasing use of mobilized peripheral blood graft, and unrelated and/or mismatched transplantation [13, 14]. A combination of a corticosteroid with a calcineurin inhibitor (CI) is the mainstay of initial management of acute and chronic GVHD. Durable responses with steroids are seen in less than half of the patients treated for aGVHD [15] and about 40–50 % of cGVHD depending upon severity of the disease [16]. Due to the lack of randomized controlled trials for treatment of steroid-refractory disease, there is no clear consensus on what comprises the best second- and third-line approach in the treatment of acute and chronic GVHD.
Pathophysiology
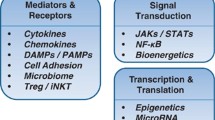
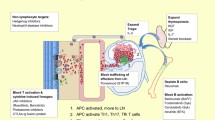
GVHD is the result of one of the fundamental functions of our immune system, i.e., identifying self from non-self. According to Billingham et al. [7], for the patient to develop GVHD, the graft should include immunologically competent cells, the host must have antigens that the donor cells would recognize as foreign leading to their activation and finally the host must be incapable to mount a response against graft cells allowing them sufficient time to attack the host tissues. Our understanding of GVHD comes mainly from animal models [17, 18]. On the basis of these models and other experiments a three-step pathogenesis of GVHD is described. Initial step is the activation of the antigen presenting cells (APC) which leads to donor T cell activation, proliferation, differentiation and migration leading to destruction of target tissues. Multiple factors affect these steps as shown in Fig. 1.
The activation of the APCs is mediated by the underlying disease process and the conditioning regimen through tissue damage, the damage to host tissues leads to production of proinflammatory cytokines [e.g., tumor necrosis factor (TNF) α, interleukin (IL) 1, 2 and 6, etc.], chemokines and increased expression of adhesion molecules, costimulatory molecules and major histocompatibility complex (MHC) antigens on the tissue [19–21]. It has been shown that increased levels of TNFα receptor after HSCT correlate directly with subsequent development of GVHD [22]. The injury to the gastrointestinal tract from the conditioning regimen also plays an important role in activation of APCs via translocation of proinflammatory stimuli such as bacterial lipopolysaccharide. The first interaction between activated host APCs and donor T cells likely takes place in the lymphoid tissues associated with gastrointestinal tract (Peyer’s patches) [23]. For the same reason reduced intensity conditioning causes less aGVHD as there is decreased damage from the conditioning regimen to the host tissue leading to less activation of the immune cascade [24, 25]. In non-clinical models GVHD can be reduced via manipulation of APCs as well [26, 27]. Also non-hematopoietic cells such as mesenchymal stromal cells have been shown to decrease the activity of alloreactive T cells leading to reduced GVHD although the mechanism of such interaction is poorly understood at this time [28]. The concept of enhanced APC activation leading to increased aGVHD explains how increased risk of GVHD is associated with more advanced stages of malignancies, more intense conditioning regimens and viral infections. The APCs detect cells infected with viral element via toll-like receptor on their surfaces which recognize viral DNA or RNA on the surface of the cells leading to activation of APCs and increasing GVHD [29]. This potentially explains the fact that viral infections such as cytomegalovirus (CMV) may trigger GVHD [30].
The second step in the process (Fig. 1) is the main step in pathogenesis of GVHD. In this step the donor T cell are activated by APCs and then differentiate and proliferate. This is aided by the expression of costimulatory molecules on the surface of APCs [31]. In animal models where genetic expression of HLA molecules can be precisely controlled, CD4+ T cells produce GVHD in response to MHC II differences while CD8+ cells do the same for MHC I differences [32, 33]. In HLA identical HSCT the GVHD is thought to be produced by CD4+ and CD8+ cells in response to minor histocompatibility antigen differences. Regulatory T Cells (CD4+, CD25+) (Tregs) have been shown to downregulate the alloreactivity of T cells in vitro and in vivo [34]. Natural killer cells (NK cells), particularly subset 1.1+ have been shown to modulate GVHD in a clinical trial. The upregulation of this subset was associated with reduced incidence of GVHD [35, 36]. Activation of the immune cells lead to transcription of genes leading to increased production of cytokines and their receptors. TH1 cytokines namely interferon-α, IL2 and TNFα are abundant in tissues with aGVHD. IL2 has been a target of interest for the treatment and prevention of GVHD [37]. IL2 is also shown to play an important role in the generation and maintenance of Tregs thus prolonged interference of IL2 may inhibit the development of long-term tolerance after allogeneic HSCT [38]. Interferon γ plays multiple roles and can both activate and/or reduce GVHD [39, 40]. It can boost GVHD by increasing the production of proinflammatory molecules and also by increasing the sensitivity of macrophages to inflammatory stimuli [41]. Decreasing production of interferon γ and increasing production of IL4 by T cells have been shown to attenuate GVHD in preclinical models [42]. Interferon γ may activate GVHD by direct damage to the GI tract epithelium and causing immunosuppression via increased production of nitric oxide [43]. Paradoxically it may reduce GVHD by accelerating apoptosis of activated T cells [44]. Transforming growth factor (TGF) β and IL10 also have regulatory roles in GVHD [45, 46].
The third step in the GVHD pathophysiology is the effector phase. It is a complex process mediated by cellular and chemical agents [47, 48]. The cellular effectors are mainly cytotoxic T cells [21]. The perforin and granzyme pathways are used by cytotoxic T cells in the development of GVHD of gastrointestinal tract while the Fas and FasL pathway is preferentially used in GVHD of liver [49]. Chemokines direct T cell migration to the target organs where they cause damage. Macrophage inflammatory protein 1 alpha and other chemokines (such as CCL2-CCL5, CXCL2, CXCL9, CXCL0, CXCL11, CCL17 and CCL27) are overexpressed and enhance localization of effector cells in experimental GVHD [50]. Expression of integrins and their respective ligands play an important role in homing of donor T cells to Peyer’s patches during aGVHD [51, 52]. Microbial products that leak through mucosal damage can stimulate secretion of inflammatory cytokines through toll-like receptors [21, 53]. The GI tract is especially susceptible to damage from TNFα and the GI tract play a major role in generation of the cytokine storm that is the characteristic of aGVHD [21]. TNFα can be produced by both donor and host cells and produces myriad of effects including activation of APCs and alloantigen presentation, localization of immune effector cells to the target organs via increased chemokine production and causing direct tissue necrosis [54–56].
The pathophysiology of cGVHD is more complex. All the previously mentioned mechanisms are relevant as well as other potential pathways. Thymic dysfunction caused by aGVHD has been implicated in development of cGVHD [57]. The presence and role of auto antibodies is also described along with implication of Treg dysfunction in the development of cGVHD [58]. A newer role of B cells including immune regulation and immunostimulation via antigen presentation has been recognized in development of cGVHD [59]. Patients with cGVHD have been found to have auto antibodies, but it is unclear whether these autoantibodies are directly pathogenic or are merely markers of B cell dysregulation [59]. Antibodies to platelet-derived growth factor (PDGF) receptor have been found in patients with scleroderma and cGVHD, also antibodies to extracellular matrix protein 1 have been found in patients with lichen sclerosis [60, 61]. Antibodies to Y chromosome mHA have been found in cGVHD patients as well, the levels of which are shown to be reduced with rituximab therapy [62]. Multiple other auto and allo antibodies have been identified in patients with cGVHD [60] but the clear function of these antibodies in pathogenesis of cGVHD as thy have in other autoimmune diseases is unclear, and they possibly represent immune dysregulation which is a hallmark of GVHD.
As described earlier Tregs play important roles in the modulation of acute and chronic GVHD. The CD4+ CD25+ Tregs have been shown to suppress proliferation and function of T cells especially TH1 cells which are the main effector of GVHD [63]. In murine model, it has been demonstrated that the incidence and severity of cGVHD is higher in the absence of recipient Tregs, and the subsequent repletion with donor or host Tregs resulted in a protective effect [64]. In addition, monitoring of FOXP3 expression as a marker of Tregs showed Treg deficiency in cGVHD patients [65]. Several studies have suggested a possible collaboration of B and T cells in the pathogenesis of cGVHD. In animal model it has been demonstrated that both donor CD4+ T and B cells are essential for development of cGVHD [66].
There is a large body of evidence regarding the role of dendritic cells in the pathogenesis of GVHD. Early donor dendritic cell reconstitution has been associated with decreased incidence of severe GVHD [67, 68]. From day 100 onwards after allogeneic HSCT the persistence of host dendritic cells correlates with onset of severe aGVHD and cGHVD [69, 70]. Modified dendritic cells with capacity to regulate immune response known as regulatory dendritic cells have a protective effect against cGVHD which is mediated by generation of alloreactive Tregs [71, 72].
Treatment of acute GVHD
aGVHD classically affects skin, liver and gastrointestinal tract. It is staged and graded based on the degree of organ involvement and clinical status of the patient [73]. The clinical feature and staging and grading of aGVHD are described in Tables 1 and 2, respectively. It is established that the overall grade of aGVHD has major impact on outcomes post HSCT, with transplant-related mortality ranging from 28 for stage 0 to 92 % for stage IV disease [74]. aGVHD can occur any time around engraftment to day 100 or so, but most likely develops in second month after allogeneic HSCT during CI-based prophylaxis [75].
First-line treatment of acute GVHD
Steroid and CI remain the gold standard for initial treatment of aGVHD. Mild skin aGVHD (grade I) can be treated with topical steroids alone. For more severe disease or any visceral involvement (grade II–IV) high-dose systemic steroid and CI are the mainstay of treatment. Studies using multiple different doses, schedules and duration of treatment have been published. In a retrospective study of 740 patients treated for grade II–IV aGVHD, 531 patients were treated with steroid and complete or partial responses were achieved in 44 % patients with improvement in skin, liver and gut disease at 43, 35 and 53 %, respectively [8]. Similar results have been seen in other retrospective studies as well [15]. The response to initial treatment correlates directly with post-transplant survival [76, 77]. The treatment for grade II–IV aGVHD is usually started with methylprednisolone at 2 mg/kg/day with CI. An exception is the aGVHD of the upper GI tract which presents with symptoms of anorexia, nausea/vomiting and dyspepsia that is more responsive to lower doses (1 mg/kg) of methylprednisolone/prednisolone. Also in skin GVHD treatment steroid is being started often at a lower dose. In gut GVHD, steroid and CI are usually started with IV due to a concern for appropriate absorption of oral medications. Higher doses of steroids have been tested in treatment of aGVHD. In a prospective study methylprednisolone 2 mg/kg/day was compared with 10 mg/kg/day. No difference in response rates, progression from grade II to III or IV or overall survival was observed [78]. In a retrospective study compared methylprednisolone 1 vs 2 mg/kg/day, no difference was seen in outcomes of patients with grade I or II aGVHD, but this study was limited by small numbers of patients with grade III and IV aGVHD [79].
Treatment with steroids especially at higher doses can lead to significant side effects including immunosuppression, hyperglycemia and osteopenia. Very few studies have evaluated effects of short vs long taper of steroids. A prospective randomized trial including 30 patient compared taper of steroids over a period of 86 vs 147 days after initial response to treatment. The short taper arm achieved resolution in 42 vs 30 days for long taper arm. No difference was observed in toxicity of steroids, development of cGVHD or 6-month overall survival [80].
Authors usually start methylprednisolone intravenously at 2 mg/kg/day, continue at that dose between 1 and 2 weeks depending on the response, then if the patient responds well to the steroid, taper down to 1.5 mg/kg/day for 1 week, 1 mg/kg/day for 1 week, then continue to taper at the rate of 10 mg/week. We often use even slower taper at doses lower than 30 mg. If initial response to steroid is not ideal, introduce a secondary agent, and taper 10 % or 10 mg every week from 2 mg/kg/day dose. The rate of the taper later on depends on the response.
Many agents in addition to steroid and CI have been evaluated for initial treatment of aGVHD, but most of them have failed to show significant benefit. In a large-scale phase II trial conducted by BMT Clinical Trial Network (BMT-CTN) patients were randomized into 4 arms to receive methylprednisolone 2 mg/kg/day and CI in addition to either etanercept, mycophenolate (MMF), denileukin or pentostatin as the initial therapy. Complete response rates at 28 days were 26, 60, 53 and 38 %, respectively, with overall survival of 47, 64, 49 and 47 % at 9 months [81]. Based on these encouraging results, a randomized phase III trial of steroid and CI with MMF vs steroid and CI has started (BMT CTN Study 0802), but the study was terminated as preliminary results did not show any difference with addition of MMF [82]. Other agents such as basiliximab, daclizumab, antithymocyte globulin (ATG), etanercept and infliximab have also been tested without convincing results [83–87]. Based on these findings, the addition of agents to high-dose steroids in first-line treatment is only recommended in the setting of clinical trials.
Treatment of steroid-refractory acute GVHD
The criterion for steroid-refractory acute GVHD is not well defined. It is generally recommended that if aGVHD worsens in any organ during the first 3 days of high-dose steroid treatment or if there is no response during the first 5–14 days second line of therapy should be considered [88]. We generally use the 3-day criterion for lower GI GVHD and introduce secondary agents by fifth day. The decision to add second-line treatment should be made sooner for patients with more severe GVHD and also in patients who cannot tolerate high-dose steroid treatment. Multiple agents have been tested for the treatment of steroid-refractory aGVHD. Unfortunately none of the existing treatments provided convincing evidences for long-term benefits. Thus, the outcome of steroid-refractory aGVHD remains poor with mortality as high as 80 % [76].
Antithymocyte globulin (ATG)
Multiple retrospective studies have shown benefit of ATG in steroid-refractory disease. The benefit is significant when used early especially in skin involvement [75]. The benefit of ATG in overall survival is yet to be shown. In a prospective randomized trial, 61 patients with aGVHD refractory to 2 mg/kg/day of methylprednisolone were treated with 5 mg/kg/day methylprednisolone alone or in combination with rabbit ATG. There was no difference between the two arms in terms of response rates, survival or TRM [89].
Alemtuzumab (Campath)
Alemtuzumab is a humanized monoclonal antibody to CD52 (a pan lymphocyte cell surface marker). In a prospective study of 18 patients with steroid-refractory aGVHD, alemtuzumab 10 mg daily was administered subcutaneously for 5 days. On day 28 of treatment 15 patients had responses and 10 out of the 15 patients were alive at 11 months. Fourteen patients developed infections including 11 who developed CMV reactivation [90]. In another phase II trial of 10 patients with grade III and IV aGVHD, 5 responded to treatment but all 10 died with a median period of 40 days [91]. Alemtuzumab is a very potent antibody but immunosuppression is very strong and life-threatening infections occur. Thus caution should be taken not to use too high dose and it should be introduced earlier than later in the course.
Anti-interleukin 2 receptor antibodies
Daclizumab and basiliximab are monoclonal antibodies directed against IL2 receptor. They have been tested in the treatment of aGVHD in the initial treatment as well as steroid-refractory setting. In a phase II study, daclizumab was given as single second-line agent to 62 patients with steroid-refractory aGVHD. Sixty-nine percent of patients achieved complete responses. Unfortunately most of the patients went on to develop severe cGVHD [92]. In another trial, 12 patients were treated with daclizumab alone or in combination with infliximab. Patients continued to receive cyclosporine and mycophenolate which was initially started as prophylaxis and they were also treated prophylactically with IV antifungal and antibacterial agents. The 200-day mortality was 17 % compared to 89 % in historical matched cohort of 12 patients treated with ATG and MMF [93]. Based on this encouraging data daclizumab was used in a trial for initial treatment for aGVHD along with steroids. The study was terminated early when the interim analysis showed worse survival for the combination arm at 100 days and 1 year [84]. This was thought to be a result of depletion of Tregs and their regulatory role in aGVHD. Other IL2 antibodies are in clinical trials as well [94, 95]. Basiliximab is a shorter acting IL2 receptor antibody. It has been associated with modest responses when used in treatment of aGVHD [95].
Anti-TNFα agents
As described earlier, TNFα plays a critical role in pathogenesis of aGVHD. It is involved in the activation of APCs, localization of effector cells to the affected tissues and cellular apoptosis. Although there are several ways to inhibit TNFα, most of the clinical trials have used either etanercept, a soluble dimeric TNFα receptor 2 that competes for TNFα binding with cellular receptors, or infliximab, a monoclonal antibody that binds to TNFα. A major difference between etanercept and infliximab is that infliximab can induce systemic elimination of monocytes and macrophages that express membrane-bound TNF, whereas etanercept does not [96]. In a retrospective study, infliximab has been shown to be associated with significant response although the proportion of patients with grade III–IV aGVHD was low and treatment was complicated by infections particularly aspergillus which could be explained by elimination of monocytes–macrophages by infliximab [97, 98]. Etanercept also increased infections in clinical trials but not as much as infliximab [81, 86]. In a phase III randomized trial of high-dose corticosteroids with or without infliximab including 63 newly diagnosed GVHD patients, no statistically significant difference was found in GVHD-related mortality, non-relapse mortality or overall survival [87]. In a study of 13 patients with acute GVHD, etanercept was shown to induce responses in 6 patients with maximal benefit seen in patients with GVHD of the gastrointestinal tract [99]. Other small studies have also shown benefit of TNFα inhibitors in treatment of aGVHD [100–102]. Combination therapy has shown to be effective as well. A study of 22 pediatric patients of steroid-refractory aGVHD who were treated with a combination of daclizumab and infliximab response was seen in 19 out of 22 patients [103]. Taken together, the published literature suggests that treatment with TNFα inhibitors is associated with improved responses in steroid-refractory aGVHD, particularly the ones involving gastrointestinal tract.
Extracorporeal photopheresis (ECP)
The majority of experience with extracorporeal photophoresis is in treatment of cGVHD. The treatment consists of a combination of leukapheresis and photodynamic therapy. The patient’s blood is exposed to 8-methoxypsoralen followed by ultraviolet A radiation before being reinfused. This process induces apoptosis of leukocytes leading to their phagocytosis by APCs and a potential switch in activity of APCs in favor of immunomodulation. In a prospective phase II trial of patients with steroid-refractory aGVHD, ECP was done weekly until maximal disease response. CR rate was 82, 61 and 61 % for aGVHD of skin, liver and GI tract, respectively. Transplant-related mortality was only 14 % in patients treated with ECP while 73 % in patients who were not [104]. Other retrospective studies have also shown benefit of ECP in treatment of aGVHD [105]. ECP is safe, without any increase in rate of infections, secondary malignancies or mortality [106].
Mycophenolate mofetil (MMF)
MMF works by inhibition of purine synthesis in lymphocytes. It is available in both oral and IV forms. There have been multiple published studies, both retrospective and prospective, using MMF in the treatment of steroid-refractory aGVHD [81, 107]. In one study it was associated with responses in 9 out of 19 patients, but this did not translate into long-term overall survival [108].
Sirolimus
Sirolimus is a mammalian-target-of-rapamycin (mTOR) inhibitor which has been used in the treatment of steroid-refractory aGVHD as well as in GVHD prophylaxis studies [109, 110]. Concerns have been raised over potential side effects of sirolimus which could include seizures, hyperlipidemia, thrombotic microangiopathy and myelosuppression. In a study of 21 steroid-refractory grade III/IV aGVHD patients, treatment with sirolimus was associated with responses in 57 % patients (CR 24 %), but treatment was discontinued in 10 patients due to no response in GVHD or toxicity [109]. Similar results were observed in retrospective studies as well [110]. It should be noted that in the GVHD prophylaxis study conducted by BMT-CTN which compared sirolimus/tacrolimus combination with methotrexate/tacrolimus combination, the option using busulfan/cyclophosphamide as conditioning regimen in sirolimus/tacrolimus arm was closed due to excessive occurrence of veno-occlusive disease (VOD) [111].
Pentostatin
Pentostatin is a nucleotide analog and is used in the treatment of lymphoid malignancies due to its anti-lymphocyte activity. In a phase I trial of pentostatin in the treatment of steroid-refractory aGVHD, out of 23 enrolled patients CR was observed in 14, but median survival was only 85 days [112]. These patients were already treated with multiple other lines of treatment for aGVHD. Another retrospective study of 13 patients has reported overall response rates of greater than 50 % [113].
Mesenchymal stem cells (MSC)
The first treatment with MSC was attempted in 2004 in a 9-year-old boy with haploidentical third party MSC [29]. Since then multiple phase I and II studies have been published using MSCs in the treatment of steroid-refractory aGVHD. The MSCs are helpful in the treatment of aGVHD due to their immunomodulatory properties [114]. In a non-randomized phase II trial of 55 patients with steroid-refractory aGVHD, use of HLA identical, haploidentical or HLA-unmatched donor MSCs was associated with CR in 30 patients and improvement in 9 additional patients [28].
How we treat steroid-refractory gut GVHD
When patient develops gut GVHD, the patient should be placed NPO, and medications should be changed to IV as much as possible, particularly CI, for the concern of appropriate absorption. Usually TPN (total parenteral nutrition) is started at this point. Methylprednisolone IV 2 mg/kg/day should be started, usually divided into twice a day doses. Prophylaxis for bacterial, fungal, Pneumocystis jerovicii and viral infections (acyclovir) should be initiated, or continued if the patient is already on them. For someone who is on high dose of steroid (more than 0.5 mg/kg/day), we usually send surveillance blood cultures (at least once a week) and viral PCRs for CMV, HHV6, adenovirus, EBV as necessary.
Particularly for gut GVHD, if the patient does not respond to high-dose steroid for 3 days, we would start infliximab 5–10 mg/kg weekly × 4 doses. We may use octreotide as necessary [115]. We would start ECP in these cases and would not add any further immunosuppression and wait until the patient responds. We also often add budesonide orally 3–6 mg daily to 3 times daily. Budesonide is supposed to be non-absorbed, but we have observed significant blood steroid levels in some cases on budesonide (unpublished observation), thus we would recommend to check the steroid level for these cases.
When the patient is responding to the treatment, we would taper steroids first. Usually for these cases we maintain 2 mg/kg for 2 weeks then start tapering 10 % weekly. But we hold off the taper if diarrhea volume is more than 500 ml/24 h and watery. When stool volume is less than 500 ml/day and contains some consistency and getting “pudding-like”, we initiate PO intake, first with clear liquid, then full liquid, then step up the diet very carefully, adding one food item in a day from bland food items we chose as “GVHD diet”. Help from nutritionists is indispensable. Fat, protein, and dairy products may predispose to diarrhea, so these are food items added last.
Treatment for steroid-refractory gut GVHD is a long, painful process, and has high mortality, but we may be able to save some of these patients by treating them very carefully.
Treatment of chronic GVHD
The organs commonly affected by cGVHD include skin, eyes, mouth, liver, gastrointestinal tract, lungs and genitalia. It is classified as mild, moderate or severe according to the NIH consensus criteria [10]. The response to treatment in cGVHD is unpredictable. Mixed responses are seen in different organs in the same patient. The risk factors of development of cGVHD are similar to aGVHD. The impact of cGVHD on survival must be considered in balance with the fact that cGVHD is associated with lower risk of relapse in leukemia (GVL effect). The correlation between GVHD severity and relapse is unclear [13, 116, 117]. The main clinical features are mentioned in Table 3.
First-line treatment of cGVHD
Patients with mild cGVHD often respond to topical treatment with corticosteroids, while systemic therapy is usually needed for treatment of moderate-to-severe disease [10]. Corticosteroids alone or in combination are the first line of systemic treatment, usually started at 1 mg/kg/day of prednisolone. There is no convincing evidence that higher doses add more benefit. The duration of treatment depends upon response to treatment and often is prolonged with median duration 2–3 years [118]. Addition of CI to steroids was shown to be beneficial in earlier studies. In a more recent randomized trial cyclosporine (CSA) (a CI) was used with or without steroids in the first-line treatment of cGVHD. No significant difference was observed in TRM, progression to secondary therapy or duration of immunosuppression. The rate of avascular hip necrosis was lower in CSA arm suggesting potential role in decreasing steroid-related side effects [16]. Other agents including azathioprine, MMF and thalidomide have failed to improve results of primary treatment of cGVHD when added to steroids and are associated with increased mortality [119]. In a phase II study, bortezomib in combination with prednisone have been associated with overall response rate of 80 % with very little toxicity [120].
Second-line treatment of cGVHD
The definition of response to treatment of cGVHD is not well characterized. It is suggested that progression of cGVHD despite 1 mg/kg/day of corticosteroids for 2 weeks or lack of improvement in symptoms after 4–8 weeks of continuous therapy or inability to taper corticosteroids should be considered as refractory disease [121]. The endpoints for the treatment of cGVHD is subjective and some of the effects of cGVHD are irreversible. There are no standard treatments for steroid-refractory cGVHD. In addition, continuing more than 20–30 mg/day of prednisolone for more than several months is associated with significant toxicities, thus many agents have been tested for steroid-sparing effect.
Rituximab
As described in the “Pathophysiology” section, B cells play a significant role in cGVHD. Rituximab, which is a monoclonal chimeric antibody to B cell surface antigen CD20, has shown activity in GVHD. Cutler et al. [122] reported a response rate of 70 % with rituximab in treatment of steroid-refractory cGVHD. In another meta-analysis involving 111 patients a cumulative response rate of 66 % was observed with rituximab [123]. Responses with rituximab were mainly partial and were limited in skin and musculoskeletal disease. A recent small prospective study evaluated combination of rituximab with alemtuzumab in 15 patients with steroid-refractory cGVHD [124]. The overall response rate was 100 % with 5 patients achieving complete response. Rituximab has potential role in reducing the irreversible damage associated with cGVHD. Rituximab therapy may have a potential role in prophylaxis for cGVHD as well [125, 126]. Various trials testing it in first-line setting are underway.
Extracorporeal photopheresis (ECP) and PUVA
ECP has been extensively evaluated in treatment of cGVHD. In a randomized multicenter trial of 95 patients with steroid-refractory or steroid-dependent cGVHD, ECP was done in addition to standard therapy [127]. Although the study did not meet its primary endpoint of total skin score (TSS) improvement, the patients in ECP arm did better in terms of steroid dose and TSS although not statistically significant. Other retrospective studies have shown benefit of ECP in treatment of cGVHD as well [106, 128]. A study of 80 patients receiving two consecutive ECP treatments every 2 weeks showed that 84 % patients were able to complete 6 months of treatment and 50 % patients had reduction in symptoms [129], thus suggesting this probably is an effective regimen. Some biomarkers were proposed which may predict the response to ECP. One study suggested role of relative levels of CD19+CD21− immature B cell [130]. Another report suggested that circulating BAFF early during therapy with ECP is an easily measured marker which may predict treatment outcome [131]. PUVA is on the same principle as ECP, but using direct irradiation to the skin, thus effective only for skin cGVHD [132], but it may be very effective in selected cases.
Imatinib
There is emerging role of TKIs especially imatinib in the treatment of cGVHD. Their actions are mainly by reducing the amount of fibrosis in conjunction by counteracting effects of TGFβ and platelet-derived growth factor (PDGF). These findings are supported by the presence of agonistic antibodies found in cGVHD to the receptors of these cytokines [133]. In a phase I/II trial, imatinib at 100 mg/day was used to treat 19 patients, both adult and pediatric, who had refractory sclerotic cGVHD of skin, gastrointestinal tract, or cGVHD of lungs. A 79 % overall response was observed at 6 months with 7 complete remissions and 8 partial responses. Toxicities observed were mainly fluid retention and myelosuppression [134]. Another small pilot study enrolling 9 patients has suggested that imatinib is helpful mainly in patients with mild lung cGVHD [135]. A study evaluating higher dose (400 mg/day) of imatinib showed response rates of about 50 % in severe cGVHD, but was associated with increased toxicity [136]. A small retrospective study has demonstrated activity of dasatinib as well [137]. The treatment with TKIs appears to be effective particularly in refractory sclerotic cGVHD.
Mycophenolate mofetil (MMF)
MMF was being increasingly used in salvage therapy for cGVHD. It was reported to have response rate of 45 % [138]. A retrospective study of de novo and steroid-refractory cGVHD has shown response rates of 90 and 75 %, respectively [139]. In a randomized prospective trial of MMF vs placebo in addition to other treatment for cGVHD, the study was terminated early due to no difference in response rate in control and study arms [140]. Common side effects of MMF include cytopenias, infections and gastrointestinal toxicity which can mimic aGVHD. Since that study MMF has been less commonly used in the treatment of cGVHD.
Sirolimus
Sirolimus has been used in combination with other agents for the treatment of cGVHD. In a phase II randomized trial 35 patients were treated with sirolimus in combination with tacrolimus and corticosteroids for steroid-refractory cGVHD [141]. Overall response rate was 63 %. Another retrospective study of patients with severe sclerodermatous cGVHD treated with sirolimus showed a response rate of 76 % [142]. Toxicities included thrombotic microangiopathy and renal dysfunction. Other small studies have shown similar results [143]. It is recommended to monitor patients for renal function, hyperlipidemia, myelosuppression particularly thrombocytopenia and thrombotic microangiopathy while on treatment with sirolimus.
It was shown that sirolimus preserves Tregs while CIs suppress Tregs [144, 145]. For that reason sirolimus is increasingly used for the treatment of CI-refractory cGVHD, by tapering off CIs while gradually increasing the dose of sirolimus to achieve the therapeutic levels.
It should be noted that sirolimus has significant interaction with many other drugs. Voriconazole may increase sirolimus level up to tenfold, while voriconazole increases tacrolimus level only twofold; thus, it is very important to check sirolimus level particularly in patients on azoles.
Pentostatin
With significant responses in steroid-refractory aGVHD, pentostatin has also been tested for steroid-refractory cGVHD. A phase II study of 58 patients with refractory cGVHD who were given pentostatin every other week for a median of 12 doses reported an overall response rate of 55 %, despite that most patients were heavily pretreated [146]. Similar results have been observed in retrospective studies as well. Infections are the most common complications.
Interleukin 2 (IL-2)
Interleukin 2 is a T cell-derived cytokine that plays a critical role in Treg development. Tregs act as immune modulators and adoptive transfer of Tregs have shown to reduce acute GVHD [147]. The clinical benefit of Treg transfer in suppressing GVHD is dependent upon in vivo expansion of transferred cells [148]. Low-dose IL-2 has recently been shown to perform this task of Treg expansion, even without Treg transfer [149]. In this study, IL-2 was administered daily for 8 weeks, partial responses were seen in 12 out of 23 evaluable patients, probability and magnitude of response was proportional to the duration of treatment. Patients also had improvement in advanced fibrotic and sclerotic manifestations of cGVHD which were previously thought to be irreversible. Responses coincided with marked expansion of Tregs. This is an exciting new strategy and needs further investigation.
Methotrexate
With efficacy in treatment of autoimmune diseases there is a potential role for methotrexate at low dose in treatment of steroid-refractory cGVHD. In a study of 86 patients with cGVHD, a marked benefit in cutaneous disease was observed with low-dose methotrexate as the first-line treatment in combination with other immunosuppressants [150]. Other smaller studies have also shown efficacious results as well [151, 152].
Bortezomib
Bortezomib is a proteasome inhibitor and is shown to induce apoptosis to alloreactive T cells in vitro by activation of caspases and cleavage of antiapoptotic protein bcl-2 [153]. In a retrospective study of 37 patients with multiple myeloma treated with reduced intensity allogeneic HSCT, 11 patients showed responses with 3 responses in patients with severe cGVHD. Eight patients with limited disease did not require any additional immunosuppressive therapy [154]. Other trials have also shown activity of bortezomib as both preventative and treatment measure for GVHD [120, 155].
Thalidomide
Thalidomide has multiple effects of immune modulation. It is known to inhibit IL6 and IL12, decrease expression of TNFα and surface adhesion molecules, and decrease angiogenesis. Vogelsang and colleagues reported complete or partial response in 14 of 44 and 12 of 44 patients with refractory cGVHD treated with thalidomide therapy and subsequent studies have produced similar results [156]. Treatment was associated with frequent discontinuation due to toxicity such as neutropenia and neurologic side effects [157].
Practical tips to treat chronic GVHD
Severe sclerotic skin cGVHD
Again, initial treatment is usually a combination of steroid and CI. CI may be replaced with sirolimus as stated above. Rituximab and ECP should be introduced relatively early. Imatinib is often very effective, but bone marrow suppression may be a problem. We usually start at a low dose, sometimes as low as 100 mg every other day, but higher dose may be more effective. So the dose should be increased as the patient can tolerate. Physical therapy to keep the joints loose and to keep the activity up is a very important part of the treatment. Patients with sclerotic skin GVHD usually have impaired body temperature control due to impaired sweating. Thus patient should be careful to stay in a well air-conditioned room and keep taking a lot of water in summer to avoid heat shock.
Patients may develop blisters and skin infections. In this instance, oral antibiotics (such as doxycycline) and local antibiotics (such as mupirocin) may be useful. Also, patients often develop skin cancers, particularly if they are also on voriconazole [158] so if they develop suspicious lesions, dermatology consult must be pursued.
Oral GVHD
We use dexamethasone rinse (0.5 mg/5 ml) 2–4 times a day (instruct the patient to spit out after rinse, as it may be too much systemic steroid if they swallow it) followed by nystatin swish. Occasionally we use clobetasol gel to be applied on the erosive lesions. Also we use tacrolimus elixir or sirolimus syrup instead of pills to provide respective medications, and instruct the patients to swish in the mouth before they swallow them.
Eye GVHD
Most of the patients develop dry eyes, thus artificial tears without preservative is necessary to keep eyes moist. Tear duct plugging has been done to keep eyes as moist as possible and often works well. For more symptomatic patients, cyclosporine and/or steroid eye drop may be used, but cyclosporine eye drop may irritate the eyes. Eye drops made of autologous serum has been tried and very effective in some cases [159]. Scleral contact lenses, a large size contact lens which rests on sclera and creates a tear-filled vault over the cornea, may help in refractory cases.
Lung chronic GVHD
In typical cases of bronchiolitis obliterans (BO) very few effective treatments are available [160]. Immune suppression with steroid may work partially, but not for a long time, thus steroid should be tapered as much as the patient can tolerate. Pulmonary rehabilitation is helpful, and providing support for these patients to change their lifestyle is necessary. For severe cases, lung transplant may be the only option.
Other support for cGVHD patients
Many patients with cGVHD may be working or would like to be back to work. It is necessary to support these patients to maintain or find jobs, and this should be done in collaboration with social workers. In addition, we should be aware of the transformed self-images particularly female patients with skin GVHD and/or with steroid effect and provide appropriate support including mental aspect. Also, many patients cannot perform as much as he/she could before GVHD, thus providing help to accept the situation and set up a new goal is important.
Future directions
Clearly there is a pressing need for evaluation of newer strategies and/or retesting of older treatment in novel settings to improve outcomes in this difficult-to-treat group of GVHD patients. All therapeutic agents currently used are associated with significant relapse and failure rates. Multiple new agents are being tested for steroid-refractory GVHD. IL6 is a potentially viable target for treatment of GVHD. IL6 increases circulating TH1 and TH17 T cell subsets and suppresses Tregs. In preclinical studies IL6 blockade has been associated marked responses in GVHD [161, 162]. Tocilizumab when used for steroid-refractory GVHD has shown responses and prophylaxis with tocilizumab is associated with markedly reduced GVHD [163]. Other agents such as vorinostat, a histone deacetylase inhibitor, have demonstrated responses [164]. Maraviroc, a CCR5 chemokine receptor inhibitor, is also being tested [165]. Adaptive transfer of Tregs has also shown to reduce GVHD [166]. Novel strategies of prevention GVHD may be more effective as compared to treatment of acute or chronic GVHD. Recent trials with the use of post-transplant cyclophosphamide in unrelated donor transplant have shown mixed results [167], but it was shown to be very effective in haploidentical donor HSCT. Inducible caspase 9 (iC9) suicide gene expressing T cells have been used to decrease incidence of GVHD and improve immune reconstitution and has shown promising results [168]. Various other agents, including ibrutinib [169], are being explored in different stages of development at this time.
Conclusions
Acute and chronic GVHD are potentially lethal complications and continues to limit survival in patients undergoing HSCT. In the last decade a lot has been learned regarding the mechanisms involved in the pathophysiology of GVHD. With this new understanding, novel pathways are being targeted and new agents are being developed/tested for the treatment of GVHD. Steroids nevertheless remain the cornerstone of the treatment of GVHD. Once GVHD is steroid refractory, options need to be considered with great attention paid to the type and stage/grade of GVHD, side effects profile, drug interactions and possible obstacles in administration of the treatment agents. With the current rush in new agents and new findings related to GVHD treatment, we will see a significant advancement in this field in next 5–10 years.
References
Thomas ED, Lochte HL Jr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257(11):491–6.
Pasquini MC WZ. Current use and outcome of hematopoietic stem cell transplantation. CIBMTR Summary Slides. 2013.
Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411(6835):385–9.
Russell NH, Kjeldsen L, Craddock C, et al. A comparative assessment of the curative potential of reduced intensity allografts in acute myeloid leukaemia. Leukemia. 2014.
Kekre N, Antin JH. Hematopoietic stem cell transplantation donor sources in the 21st century: choosing the ideal donor when a perfect match does not exist. Blood. 2014;124(3):334–43.
Solh M. Haploidentical vs cord blood transplantation for adults with acute myelogenous leukemia. World J Stem Cells. 2014;6(4):371–9.
Billingham RE. The biology of graft-versus-host reactions. Harvey Lect. 1966;62:21–78.
Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood. 1990;76(8):1464–72.
Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28(3):250–9.
Filipovich AH, Weisdorf D, Pavletic S, National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease, et al. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–56.
Griffith LM, Pavletic SZ, Lee SJ, Martin PJ, Schultz KR, Vogelsang GB. Chronic graft-versus-host disease–implementation of the national institutes of health consensus criteria for clinical trials. Biol Blood Marrow Transplant. 2008;14:379–84.
Jagasia MH, Greinix HT, Arora M, National Institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2014;21:389–401.
Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100(2):406–14.
Flowers ME, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to national institutes of health consensus criteria. Blood. 2011;117(11):3214–9.
MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8(7):387–94.
Koc S, Leisenring W, Flowers ME, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100(1):48–51.
Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7(5):340–52.
Socie G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114(20):4327–36.
Xun CQ, Thompson JS, Jennings CD, Brown SA, Widmer MB. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood. 1994;83(8):2360–7.
Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90(8):3204–13.
Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95(9):2754–9.
Choi SW, Kitko CL, Braun T, et al. Change in plasma tumor necrosis factor receptor 1 levels in the first week after myeloablative allogeneic transplantation correlates with severity and incidence of GVHD and survival. Blood. 2008;112(4):1539–42.
Murai M, Yoneyama H, Ezaki T, et al. Peyer’s patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat Immunol. 2003;4(2):154–60.
Couriel DR, Saliba RM, Giralt S, et al. Acute and chronic graft-versus-host disease after ablative and nonmyeloablative conditioning for allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant. 2004;10(3):178–85.
Sorror ML, Maris MB, Storer B, et al. Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative and myeloablative conditioning: influence of pretransplantation comorbidities. Blood. 2004;104(4):961–8.
Duffner UA, Maeda Y, Cooke KR, et al. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J Immunol. 2004;172(12):7393–8.
Sato K, Yamashita N, Baba M, Matsuyama T. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity. 2003;18(3):367–79.
Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–86.
Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–41.
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801.
Dustin ML. Role of adhesion molecules in activation signaling in T lymphocytes. J Clin Immunol. 2001;21(4):258–63.
Korngold R, Sprent J. Features of T cells causing H-2-restricted lethal graft-vs.-host disease across minor histocompatibility barriers. J Exp Med. 1982;155(3):872–83.
Korngold R, Sprent J. Surface markers of T cells causing lethal graft-vs-host disease to class I vs class II H-2 differences. J Immunol. 1985;135(5):3004–10.
Cohen JL, Boyer O. The role of CD4+ CD25 hi regulatory T cells in the physiopathogeny of graft-versus-host disease. Curr Opin Immunol. 2006;18(5):580–5.
Zeng D, Lewis D, Dejbakhsh-Jones S, et al. Bone marrow NK1.1(-) and NK1.1(+) T cells reciprocally regulate acute graft versus host disease. J Exp Med. 1999;189(7):1073–81.
Lowsky R, Takahashi T, Liu YP, et al. Protective conditioning for acute graft-versus-host disease. N Engl J Med. 2005;353(13):1321–31.
Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92(7):2303–14.
Zeiser R, Nguyen VH, Beilhack A, et al. Inhibition of CD4+ CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108(1):390–9.
Fowler DH, Kurasawa K, Smith R, Eckhaus MA, Gress RE. Donor CD4-enriched cells of Th2 cytokine phenotype regulate graft-versus-host disease without impairing allogeneic engraftment in sublethally irradiated mice. Blood. 1994;84(10):3540–9.
Yang YG, Dey BR, Sergio JJ, Pearson DA, Sykes M. Donor-derived interferon gamma is required for inhibition of acute graft-versus-host disease by interleukin 12. J Clin Invest. 1998;102(12):2126–35.
Reddy P, Ferrara JL. Graft-Versus-Host Disease and Graft-Versus-Leukemia Responses. In: Hoffman RB, Edward J, Edward Silberstein J, Leslie Heslop E, Helen E, Jeffrey I, Anastasi I, John, editors. Basic principles and practice. USA: Hematology; 2013. p. 1592–611.
Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006;43(1):3–10.
Krenger W, Falzarano G, Delmonte J Jr, Snyder KM, Byon JC, Ferrara JL. Interferon-gamma suppresses T-cell proliferation to mitogen via the nitric oxide pathway during experimental acute graft-versus-host disease. Blood. 1996;88(3):1113–21.
Reddy P, Teshima T, Kukuruga M, et al. Interleukin-18 regulates acute graft-versus-host disease by enhancing fas-mediated donor T cell apoptosis. J Exp Med. 2001;194(10):1433–40.
Banovic T, MacDonald KP, Morris ES, et al. TGF-beta in allogeneic stem cell transplantation: friend or foe? Blood. 2005;106(6):2206–14.
Lin MT, Storer B, Martin PJ, et al. Relation of an interleukin-10 promoter polymorphism to graft-versus-host disease and survival after hematopoietic-cell transplantation. N Engl J Med. 2003;349(23):2201–10.
Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324(10):667–74.
Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–70.
van den Brink MR, Burakoff SJ. Cytolytic pathways in haematopoietic stem-cell transplantation. Nat Rev Immunol. 2002;2(4):273–81.
Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105(11):4191–9.
Waldman E, Lu SX, Hubbard VM, et al. Absence of beta7 integrin results in less graft-versus-host disease because of decreased homing of alloreactive T cells to intestine. Blood. 2006;107(4):1703–11.
Welniak LA, Kuprash DV, Tumanov AV, et al. Peyer patches are not required for acute graft-versus-host disease after myeloablative conditioning and murine allogeneic bone marrow transplantation. Blood. 2006;107(1):410–2.
Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–95.
Hill GR, Teshima T, Rebel VI, et al. The p55 TNF-alpha receptor plays a critical role in T cell alloreactivity. J Immunol. 2000;164(2):656–63.
Piguet PF, Grau GE, Allet B, Vassalli P. Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-vs -host disease. J Exp Med. 1987;166(5):1280–9.
Brown GR, Lee E, Thiele DL. TNF-TNFR2 interactions are critical for the development of intestinal graft-versus-host disease in MHC class II-disparate (C57BL/6 J–> C57BL/6 J × bm12)F1 mice. J Immunol. 2002;168(6):3065–71.
Krenger W, Hollander GA. The thymus in GVHD pathophysiology. Best Pract Res Clin Haematol. 2008;21(2):119–28.
Martin PJ. Biology of chronic graft-versus-host disease: implications for a future therapeutic approach. Keio J Med. 2008;57(4):177–83.
Shimabukuro-Vornhagen A, Hallek MJ, Storb RF, von Bergwelt-Baildon MS. The role of B cells in the pathogenesis of graft-versus-host disease. Blood. 2009;114(24):4919–27.
Kapur R, Ebeling S, Hagenbeek A. B-cell involvement in chronic graft-versus-host disease. Haematologica. 2008;93(11):1702–11.
Chan I, Oyama N, Neill SM, Wojnarowska F, Black MM, McGrath JA. Characterization of IgG autoantibodies to extracellular matrix protein 1 in lichen sclerosus. Clin Exp Dermatol. 2004;29(5):499–504.
Bates JS, Engemann AM, Hammond JM. Clinical utility of rituximab in chronic graft-versus-host disease. Ann Pharmacother. 2009;43(2):316–21.
Cosmi L, Liotta F, Angeli R, et al. Th2 cells are less susceptible than Th1 cells to the suppressive activity of CD25+ regulatory thymocytes because of their responsiveness to different cytokines. Blood. 2004;103(8):3117–21.
Anderson BE, McNiff JM, Matte C, Athanasiadis I, Shlomchik WD, Shlomchik MJ. Recipient CD4+ T cells that survive irradiation regulate chronic graft-versus-host disease. Blood. 2004;104(5):1565–73.
Miura Y, Thoburn CJ, Bright EC, et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104(7):2187–93.
Zhang C, Todorov I, Zhang Z, et al. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107(7):2993–3001.
Auffermann-Gretzinger S, Lossos IS, Vayntrub TA, et al. Rapid establishment of dendritic cell chimerism in allogeneic hematopoietic cell transplant recipients. Blood. 2002;99(4):1442–8.
Klangsinsirikul P, Carter GI, Byrne JL, Hale G, Russell NH. Campath-1G causes rapid depletion of circulating host dendritic cells (DCs) before allogeneic transplantation but does not delay donor DC reconstitution. Blood. 2002;99(7):2586–91.
Chan GW, Gorgun G, Miller KB, Foss FM. Persistence of host dendritic cells after transplantation is associated with graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9(3):170–6.
Teshima T, Ordemann R, Reddy P, et al. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8:575–81.
Fujita S, Sato Y, Sato K, et al. Regulatory dendritic cells protect against cutaneous chronic graft-versus-host disease mediated through CD4+ CD25 + Foxp3+ regulatory T cells. Blood. 2007;110(10):3793–803.
Marcondes AM, Karoopongse E, Lesnikova M, et al. Alpha-1-antitrypsin (AAT)-modified donor cells suppress GVHD but enhance the GVL effect: a role for mitochondrial bioenergetics. Blood. 2014;124(18):2881–91.
Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304.
Gratwohl A, Hermans J, Apperley J, et al. Acute graft-versus-host disease: grade and outcome in patients with chronic myelogenous leukemia. working party chronic leukemia of the European Group for Blood and Marrow Transplantation. Blood. 1995;86(2):813–8.
MacMillan ML, Weisdorf DJ, Davies SM, et al. Early antithymocyte globulin therapy improves survival in patients with steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8(1):40–6.
Weisdorf D, Haake R, Blazar B, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990;75(4):1024–30.
Saliba RM, Couriel DR, Giralt S, et al. Prognostic value of response after upfront therapy for acute GVHD. Bone Marrow Transplant. 2012;47(1):125–31.
Van Lint MT, Uderzo C, Locasciulli A, et al. Early treatment of acute graft-versus-host disease with high- or low-dose 6-methylprednisolone: a multicenter randomized trial from the Italian Group for Bone Marrow Transplantation. Blood. 1998;92(7):2288–93.
Mielcarek M, Storer BE, Boeckh M, et al. Initial therapy of acute graft-versus-host disease with low-dose prednisone does not compromise patient outcomes. Blood. 2009;113(13):2888–94.
Hings IM, Filipovich AH, Miller WJ, et al. Prednisone therapy for acute graft-versus-host disease: short- versus long-term treatment. A prospective randomized trial. Transplantation. 1993;56(3):577–80.
Alousi AM, Weisdorf DJ, Logan BR, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the blood and marrow transplant clinical trials network. Blood. 2009;114(3):511–7.
Bolaños-Meade J, Logan BR, Alousi AM, Antin JH, Barowski K, Carter SL, Goldstein SC, Hexner EO, Horowitz MM, Lee SJ, Levine JE, MacMillan ML, Martin PJ, Mendizabal AM, Nakamura R, Pasquini MC, Weisdorf DJ, Westervelt P, Ho VT. Phase 3 clinical trial of steroids/mycophenolate mofetil vs steroids/placebo as therapy for acute GVHD: BMT CTN 0802. Blood. 2014;124(22):3221–7.
Cahn JY, Bordigoni P, Tiberghien P, et al. Treatment of acute graft-versus-host disease with methylprednisolone and cyclosporine with or without an anti-interleukin-2 receptor monoclonal antibody. A multicenter phase III study. Transplantation. 1995;60(9):939–42.
Lee SJ, Zahrieh D, Agura E, et al. Effect of up-front daclizumab when combined with steroids for the treatment of acute graft-versus-host disease: results of a randomized trial. Blood. 2004;104(5):1559–64.
Cragg L, Blazar BR, Defor T, et al. A randomized trial comparing prednisone with antithymocyte globulin/prednisone as an initial systemic therapy for moderately severe acute graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6(4a):441–7.
Levine JE, Paczesny S, Mineishi S, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood. 2008;111(4):2470–5.
Couriel DR, Saliba R, de Lima M, et al. A phase III study of infliximab and corticosteroids for the initial treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15(12):1555–62.
Dignan FL, Amrolia P, Clark A, et al. Diagnosis and management of chronic graft-versus-host disease. Br J Haematol. 2012;158(1):46–61.
Van Lint MT, Milone G, Leotta S, et al. Treatment of acute graft-versus-host disease with prednisolone: significant survival advantage for day +5 responders and no advantage for nonresponders receiving anti-thymocyte globulin. Blood. 2006;107(10):4177–81.
Gomez-Almaguer D, Ruiz-Arguelles GJ, del Carmen Tarin-Arzaga L, et al. Alemtuzumab for the treatment of steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14(1):10–5.
Martinez C, Solano C, Ferra C, Sampol A, Valcarcel D, Perez-Simon JA. Alemtuzumab as treatment of steroid-refractory acute graft-versus-host disease: results of a phase II study. Biol Blood Marrow Transplant. 2009;15(5):639–42.
Bordigoni P, Dimicoli S, Clement L, et al. Daclizumab, an efficient treatment for steroid-refractory acute graft-versus-host disease. Br J Haematol. 2006;135(3):382–5.
Srinivasan R, Chakrabarti S, Walsh T, et al. Improved survival in steroid-refractory acute graft versus host disease after non-myeloablative allogeneic transplantation using a daclizumab-based strategy with comprehensive infection prophylaxis. Br J Haematol. 2004;124(6):777–86.
Bay JO, Dhedin N, Goerner M, et al. Inolimomab in steroid-refractory acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation: retrospective analysis and comparison with other interleukin-2 receptor antibodies. Transplantation. 2005;80(6):782–8.
Schmidt-Hieber M, Fietz T, Knauf W, et al. Efficacy of the interleukin-2 receptor antagonist basiliximab in steroid-refractory acute graft-versus-host disease. Br J Haematol. 2005;130(4):568–74.
Ehlers S. Tumor necrosis factor and its blockade in granulomatous infections: differential modes of action of infliximab and etanercept? Clin Infect Dis. 2005;41(Suppl 3):S199–203.
Couriel D, Saliba R, Hicks K, et al. Tumor necrosis factor-alpha blockade for the treatment of acute GVHD. Blood. 2004;104(3):649–54.
Marty FM, Lee SJ, Fahey MM, et al. Infliximab use in patients with severe graft-versus-host disease and other emerging risk factors of non-candida invasive fungal infections in allogeneic hematopoietic stem cell transplant recipients: a cohort study. Blood. 2003;102(8):2768–76.
Busca A, Locatelli F, Marmont F, Ceretto C, Falda M. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2007;82(1):45–52.
Sleight BS, Chan KW, Braun TM, Serrano A, Gilman AL. Infliximab for GVHD therapy in children. Bone Marrow Transplant. 2007;40(5):473–80.
Patriarca F, Sperotto A, Damiani D, et al. Infliximab treatment for steroid-refractory acute graft-versus-host disease. Haematologica. 2004;89(11):1352–9.
Jacobsohn DA, Hallick J, Anders V, McMillan S, Morris L, Vogelsang GB. Infliximab for steroid-refractory acute GVHD: a case series. Am J Hematol. 2003;74(2):119–24.
Rao K, Rao A, Karlsson H, Jagani M, Veys P, Amrolia PJ. Improved survival and preserved antiviral responses after combination therapy with daclizumab and infliximab in steroid-refractory graft-versus-host disease. J Pediatr Hematol Oncol. 2009;31(6):456–61.
Greinix HT, Knobler RM, Worel N, et al. The effect of intensified extracorporeal photochemotherapy on long-term survival in patients with severe acute graft-versus-host disease. Haematologica. 2006;91(3):405–8.
Perfetti P, Carlier P, Strada P, et al. Extracorporeal photopheresis for the treatment of steroid refractory acute GVHD. Bone Marrow Transplant. 2008;42(9):609–17.
Couriel DR, Hosing C, Saliba R, et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood. 2006;107(8):3074–80.
Pidala J, Kim J, Perkins J, et al. Mycophenolate mofetil for the management of steroid-refractory acute graft vs host disease. Bone Marrow Transplant. 2010;45:91–9924.
Furlong T, Martin P, Flowers ME, et al. Therapy with mycophenolate mofetil for refractory acute and chronic GVHD. Bone Marrow Transplant. 2009;44(11):739–48.
Benito AI, Furlong T, Martin PJ, et al. Sirolimus (rapamycin) for the treatment of steroid-refractory acute graft-versus-host disease. Transplantation. 2001;72(12):1924–9.
Hoda D, Pidala J, Salgado-Vila N, et al. Sirolimus for treatment of steroid-refractory acute graft-versus-host disease. Bone Marrow Transplant. 2010;45(8):1347–51.
Cutler C, Logan B, Nakamura R, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124(8):1372–7.
Bolanos-Meade J, Jacobsohn DA, Margolis J, et al. Pentostatin in steroid-refractory acute graft-versus-host disease. J Clin Oncol. 2005;23(12):2661–8.
Pidala J, Kim J, Roman-Diaz J, et al. Pentostatin as rescue therapy for glucocorticoid-refractory acute and chronic graft-versus-host disease. Ann Transplant. 2010;15(4):21–9.
Dazzi F, Lopes L, Weng L. Mesenchymal stromal cells: a key player in innate tolerance? Immunology. 2012;137(3):206–13.
Ippoliti C, Neumann J. Octreotide in the management of diarrhea induced by graft versus host disease. Oncol Nurs Forum. 1998;25(5):873–8.
Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–62.
Gratwohl A, Brand R, Apperley J, et al. Graft-versus-host disease and outcome in HLA-identical sibling transplantations for chronic myeloid leukemia. Blood. 2002;100(12):3877–86.
Stewart BL, Storer B, Storek J, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104(12):3501–6.
Wolff D, Gerbitz A, Ayuk F, et al. Consensus conference on clinical practice in chronic graft-versus-host disease (GVHD): first-line and topical treatment of chronic GVHD. Biol Blood Marrow Transplant. 2010;16(12):1611–28.
Herrera AF, Kim HT, Bindra B, et al. A phase II study of bortezomib plus prednisone for initial therapy of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20(11):1737–43.
Martin PJ, Weisdorf D, Przepiorka D, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: vI. design of clinical trials working group report. Biol Blood Marrow Transplant. 2006;12(5):491–505.
Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108(2):756–62.
Kharfan-Dabaja MA, Mhaskar AR, Djulbegovic B, Cutler C, Mohty M, Kumar A. Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2009;15(9):1005–13.
Gutierrez-Aguirre CH, Cantu-Rodriguez OG, Borjas-Almaguer OD, et al. Effectiveness of subcutaneous low-dose alemtuzumab and rituximab combination therapy for steroid-resistant chronic graft-versus-host disease. Haematologica. 2012;97(5):717–22.
van Dorp S, Pietersma F, Wolfl M, et al. Rituximab treatment before reduced-intensity conditioning transplantation associates with a decreased incidence of extensive chronic GVHD. Biol Blood Marrow Transplant. 2009;15(6):671–8.
Arai S, Sahaf B, Narasimhan B, et al. Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood. 2012;119(25):6145–54.
Flowers ME, Apperley JF, van Besien K, et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood. 2008;112(7):2667–74.
Scarisbrick JJ, Taylor P, Holtick U, et al. UK consensus statement on the use of extracorporeal photopheresis for treatment of cutaneous T-cell lymphoma and chronic graft-versus-host disease. Br J Dermatol. 2008;158(4):659–78.
Dignan FL, Greenblatt D, Cox M, et al. Efficacy of bimonthly extracorporeal photopheresis in refractory chronic mucocutaneous GVHD. Bone Marrow Transplant. 2012;47(6):824–30.
Kuzmina Z, Greinix HT, Knobler R, et al. Proportions of immature CD19+CD2− B lymphocytes predict the response to extracorporeal photopheresis in patients with chronic graft-versus-host disease. Blood. 2009;114:744–6.
Whittle R, Taylor PC. Circulating B-cell activating factor level predicts clinical response of chronic graft-versus-host disease to extracorporeal photopheresis. Blood. 2011;118(24):6446–9.
Ghoreschi K, Thomas P, Penovici M, et al. PUVA-bath photochemotherapy and isotretinoin in sclerodermatous graft-versus-host disease. Eur J Dermatol. 2008;18(6):667–70.
Svegliati S, Olivieri A, Campelli N, et al. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood. 2007;110(1):237–41.
Olivieri A, Locatelli F, Zecca M, et al. Imatinib for refractory chronic graft-versus-host disease with fibrotic features. Blood. 2009;114(3):709–18.
Stadler M, Ahlborn R, Kamal H. Limited efficacy of imatinib in severe pulmonary chronic graft-versus-host disease, vol. 114. United States: Blood; 2009. p. 3718–9 (author reply 3719–3720).
Magro L, Mohty M, Catteau B, et al. Imatinib mesylate as salvage therapy for refractory sclerotic chronic graft-versus-host disease. Blood. 2009;114(3):719–22.
Sanchez-Ortega I, Servitje O, Arnan M, et al. Dasatinib as salvage therapy for steroid refractory and imatinib resistant or intolerant sclerotic chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2012;18(2):318–23.
Koreth J, Antin JH. Current and future approaches for control of graft-versus-host disease. Expert Rev Hematol. 2008;1(1):111.
Lopez F, Parker P, Nademanee A, et al. Efficacy of mycophenolate mofetil in the treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11(4):307–13.
Martin PJ, Storer BE, Rowley SD, et al. Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood. 2009;113(21):5074–82.
Couriel DR, Saliba R, Escalon MP, et al. Sirolimus in combination with tacrolimus and corticosteroids for the treatment of resistant chronic graft-versus-host disease. Br J Haematol. 2005;130(3):409–17.
Jedlickova Z, Burlakova I, Bug G, Baurmann H, Schwerdtfeger R, Schleuning M. Therapy of sclerodermatous chronic graft-versus-host disease with mammalian target of rapamycin inhibitors. Biol Blood Marrow Transplant. 2011;17(5):657–63.
Johnston LJ, Brown J, Shizuru JA, et al. Rapamycin (sirolimus) for treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11(1):47–55.
Shin HJ, Baker J, Leveson-Gower DB, Smith AT, Sega EI, Negrin RS. Rapamycin and IL-2 reduce lethal acute graft-versus-host disease associated with increased expansion of donor type CD4+ CD25+ Foxp3+ regulatory T cells. United States: Blood; 2011. p. 2342–50.
Peccatori J, Forcina A, Clerici D. Sirolimus-based graft-versus-host disease prophylaxis promotes the in vivo expansion of regulatory T cells and permits peripheral blood stem cell transplantation from haploidentical donors, vol. 29. England: Leukemia; 2015. p. 396–405.
Jacobsohn DA, Chen AR, Zahurak M, et al. Phase II study of pentostatin in patients with corticosteroid-refractory chronic graft-versus-host disease. J Clin Oncol. 2007;25(27):4255–61.
Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+) CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196(3):389–99.
Wolf D, von Lilienfeld-Toal M, Wolf AM, et al. Novel treatment concepts for graft-versus-host disease. Blood. 2012;119(1):16–25.
Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365(22):2055–66.
Wang Y, Xu LP, Liu DH, et al. First-line therapy for chronic graft-versus-host disease that includes low-dose methotrexate is associated with a high response rate. Biol Blood Marrow Transplant. 2009;15(4):505–11.
Inagaki J, Nagatoshi Y, Hatano M, Isomura N, Sakiyama M, Okamura J. Low-dose MTX for the treatment of acute and chronic graft-versus-host disease in children. Bone Marrow Transplant. 2008;41(6):571–7.
Huang XJ, Jiang Q, Chen H, et al. Low-dose methotrexate for the treatment of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;36(4):343–8.
Blanco B, Sanchez-Abarca LI, Caballero-Velazquez T, Santamaria C, Inoges S, Perez-Simon JA. Depletion of alloreactive T-cells in vitro using the proteasome inhibitor bortezomib preserves the immune response against pathogens. Leuk Res. 2011;35(10):1412–5.
El-Cheikh J, Michallet M, Nagler A, et al. High response rate and improved graft-versus-host disease following bortezomib as salvage therapy after reduced intensity conditioning allogeneic stem cell transplantation for multiple myeloma. Haematologica. 2008;93(3):455–8.
Koreth J, Stevenson KE, Kim HT, et al. Bortezomib-based graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor transplantation. J Clin Oncol. 2012;30(26):3202–8.
Vogelsang GB, Farmer ER, Hess AD, et al. Thalidomide for the treatment of chronic graft-versus-host disease. N Engl J Med. 1992;326(16):1055–8.
Koc S, Leisenring W, Flowers ME, et al. Thalidomide for treatment of patients with chronic graft-versus-host disease. Blood. 2000;96(12):3995–6.
Cowen EW, Nguyen JC, Miller DD, et al. Chronic phototoxicity and aggressive squamous cell carcinoma of the skin in children and adults during treatment with voriconazole. J Am Acad Dermatol. 2010;62(1):31–7.
Rocha EM, Pelegrino FS, de Paiva CS, Vigorito AC, de Souza CA. GVHD dry eyes treated with autologous serum tears. Bone Marrow Transplant. 2000;25(10):1101–3.
Yoshihara S, Yanik G, Cooke KR, Mineishi S. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(7):749–59.
Chen X, Das R, Komorowski R, et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009;114(4):891–900.
Tawara I, Koyama M, Liu C, et al. Interleukin-6 modulates graft-versus-host responses after experimental allogeneic bone marrow transplantation. Clin Cancer Res. 2011;17(1):77–88.
Drobyski WR, Pasquini M, Kovatovic K, et al. Tocilizumab for the treatment of steroid refractory graft-versus-host disease. Biol Blood Marrow Transplant. 2011;17(12):1862–8.
Choi S, Reddy P. HDAC inhibition and graft versus host disease. Mol Med. 2011;17(5–6):404–16.
Reshef R, Luger SM, Hexner EO, et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med. 2012;367(2):135–45.
Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–8.
Al-Homsi AS, Roy TS, Cole K, Feng Y, Duffner U. Post-Transplant High-Dose Cyclophosphamide for the Prevention of Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2014;21:604–11.
Zhou X, Di Stasi A, Tey SK, et al. Long-term outcome after haploidentical stem cell transplant and infusion of T cells expressing the inducible caspase nine safety transgene. Blood. 2014;123(25):3895–905.
Dubovsky JA, Flynn R, Du J, et al. Ibrutinib treatment ameliorates murine chronic graft-versus-host disease. J Clin Invest. 2014;124(11):4867–76.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Jamil, M.O., Mineishi, S. State-of-the-art acute and chronic GVHD treatment. Int J Hematol 101, 452–466 (2015). https://doi.org/10.1007/s12185-015-1785-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-015-1785-1