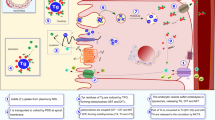
Abstract
Maternal hypothyroxinemia can induce neurodevelopmental impairments in the developing fetus. We here review recent studies on the epidemiology and molecular mechanisms associated with this important public health issue. In 2011, the American Thyroid Association defined maternal hypothyroxinemia as low serum free thyroxine (FT4) levels (<5th or <10th percentile) existing in conjunction with normal serum free triiodothyronine (FT3) or thyroid stimulating hormone (TSH) levels during pregnancy. Compared to clinical or subclinical hypothyroidism, hypothyroxinemia is more commonly found in pregnant women. Hypothyroxinemia usually ensues in response to several factors, such as mild iodine deficiency, environmental endocrine disrupters, or certain thyroid diseases. Unequivocal evidence demonstrates that maternal hypothyroxinemia leads to negative effects on fetal brain development, increasing the risks for cognitive deficits and poor psychomotor development in resulting progeny. In support of this, rodent models provide direct evidence of neurodevelopmental damage induced by maternal hypothyroxinemia, including dendritic and axonal growth limitation, neural abnormal location, and synaptic function alteration. The neurodevelopmental impairments induced by hypothyroxinemia suggest an independent role of T4. Increasing evidence indicates that adequate thyroxine is required for the mothers in order to protect against the abnormal brain development in their progeny.


Similar content being viewed by others
References
Morreale de Escobar G, Obregon MJ, Escobar del Rey F (2000) Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab 85:3975–3987
Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN et al (2011) Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 21(10):1081–1125
Finken MJ, van Eijsden M, Loomans EM, Vrijkotte TG, Rotteveel J (2013) Maternal hypothyroxinemia in early pregnancy predicts reduced performance in reaction time tests in 5- to 6-year-old offspring. J Clin Endocrinol Metab 98(4):1417–1426
Boelaert K, Franklyn JA (2005) Thyroid hormone in health and disease. J Endocrinol 187(1):1–15
Pinazo-Durán MD, Pons-Vázquez S, Gallego-Pinazo R, Galbis Estrada C, Zanón-Moreno V, Vila Bou V, Sanz Solana P (2011) Thyroid hormone deficiency disrupts rat eye neurodevelopment. Brain Res 1392:16–26
Williams GR (2008) Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol 20:784–794
Bernal J, Guadaño-Ferraz A, Morte B (2003) Perspectives in the study of thyroid hormone action on brain development and function. Thyroid 13(11):1005–1012
Cheng SY, Leonard JL, Davis PJ (2010) Molecular aspects of thyroid hormone actions. Endocr Rev 31:139–170
Chen C, Zhou Z, Zhong M, Zhang Y, Li M, Zhang L, Qu M, Yang J, et al (2012) Thyroid hormone promotes neuronal differentiation of embryonic neural stem cells by inhibiting STAT3 signaling through TRα1. Stem Cells Dev 21:2667–2681
Patel J, Landers K, Li H, Mortimer RH, Richard K (2011) Thyroid hormones and fetal neurological development. J Endocrinol 209:1–8
König S, Moura Neto V (2002) Thyroid hormone actions on neural cells. Cell Mol Neurobiol 22(5–6):517–544
Ausó E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escoba G, Berbel P (2004) A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology 145:4037–4047
Henrichs J, Ghassabian A, Peeters RP, Tiemeier H (2013) Maternal hypothyroxinemia and effects on cognitive functioning in childhood: how and why? Clin Endocrinol (Oxf) 79:152–162
Kooistra L, Crawford S, van Baar AL, Brouwers EP, Pop VJ (2006) Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics 117:161–167
Li Y, Shan Z, Teng W, Yu X, Li Y, Fan C, Teng X, Guo R et al (2010) Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clin Endocrinol (Oxf) 72:825–829
Opazo MC, Gianini A, Pancetti F, Azkcona G, Alarcón L, Lizana R, Noches V, Gonzalez PA et al (2008) Maternal hypothyroxinemia impairs spatial learning and synaptic nature and function in the offspring. Endocrinology 149(10):5097–5106
Wei W, Wang Y, Wang Y, Dong J, Min H, Song B, Teng W, Xi Q et al (2013) Developmental hypothyroxinemia induced by maternal mild iodine deficiency delays hippocampal axonal growth in the rat offspring. J Neuroendocrinol 25:852–862
Wang Y, Wang Y, Dong J, Wei W, Song B, Min H, Teng W, Chen J (2014) Developmental hypothyroxinemia and hypothyroidism limit dendritic growth of cerebellar purkinje cells in rat offspring: involvement of MAP2 and stathmin. Neuropathol Appl Neurobiol 40:398–415
Henrichs J, Bongers-Schokking JJ, Schenk JJ, Ghassabian A, Schmidt HG, Visser TJ, Hooijkaas H, de Muinck Keizer-Schrama SM et al (2010) Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab 95(9):4227–4234
Suárez-Rodríguez M, Azcona-San Julián C, Alzina de Aguilar V (2012) Hypothyroxinemia during pregnancy: the effect on neurodevelopment in the child. Int J Dev Neurosci 30(6):435–438
Moleti M, Lo Presti VP, Mattina F, Mancuso A, De Vivo A, Giorgianni G, Di Bella B, Trimarchi F et al (2009) Gestational thyroid function abnormalities in conditions of mild iodine deficiency: early screening versus continuous monitoring of maternal thyroid status. Eur J Endocrinol 160(4):611–617
de Escobar GM, Ares S, Berbel P, Obregón MJ, del Rey FE (2008) The changing role of maternal thyroid hormone in fetal brain development. Semin Perinatol 32(6):380–386
Krassas GE, Poppe K, Glinoer D (2010) Thyroid function and human reproductive health. Endocr Rev 31(5):702–755
Poppe K, Glinoer D (2003) Thyroid autoimmunity and hypothyroidism before and during pregnancy. Hum Reprod Update 9:149–161
Hu X, Teng X, Zheng H, Shan Z, Li J, Jin T, Xiong C, Zhang H et al (2014) Iron deficiency without anemia causes maternal hypothyroxinemia in pregnant rats. Nutr Res 34(7):604–612
Vermiglio F, Lo Presti VP, Moleti M, Sidoti M, Tortorella G, Scaffidi G, Castagna MG, Mattina F et al (2004) Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate ID: a possible novel ID disorder in developed countries. J Clin Endocrinol Metab 89:6054–6060
Zimmermann MB (2009) Iodine deficiency. Endocr Rev 30:376–408
Zimmermann MB, Andersson M (2012) Update on iodine status worldwide. Curr Opin Endocrinol Diabetes Obes 19:382–387
Pearce EN, Andersson M, Zimmermann MB (2013) Global iodine nutrition: where do we stand in 2013? Thyroid 23:523–528
Saira S, Khattak RM, Rehman AU, Khan AA, Khattak MNK (2014) Prevalence of goiter and iodine status in 6–12 years school children and pregnant women of district Charsadda, Pakistan. Acta Endocrinol (Buc) 10:65–75
Andersson M, Karumbunathan V, Zimmermann MB (2012) Global iodine status in 2011 and trends over the past decade. J Nutr 142:744–750
Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, Carter JA (2007) Child development: risk factors for adverse outcomes in developing countries. Lancet 369:145–157
Kerac M, Postels DG, Mallewa M, Jalloh AA, Voskuijl WP, Groce N, Gladstone M, Molyneux E (2014) The interaction of malnutrition and neurological disability in Africa. Semin Pediatr Neurol 21:42–49
Clifton VL, Hodyl NA, Fogarty PA, Torpy DJ, Roberts R, Nettelbeck T, Ma G, Hetzel B (2013) The impact of iodine supplementation and bread fortification on urinary iodine concentrations in a mildly iodine deficient population of pregnant women in South Australia. Nutr J 12:32
Bath SC, Rayman MP (2013) Iodine deficiency in the UK: an overlooked cause of impaired neurodevelopment? Proc Nutr Soc 72:226–235
Gowachirapant S, Melse-Boonstra A, Winichagoon P, Zimmermann MB (2014) Overweight increases risk of first trimester hypothyroxinaemia in iodine-deficient pregnant women. Matern Child Nutr 10:61–71
World Health Organization/UNICEF/ICCIDD (2007) Assessment of ID disorders and monitoring their elimination: a guide for programme managers, 3rd edn. World Health Organization, Geneva, Available from http://whqlibdoc.who.int/publications/2007/9789241595827_eng.pdf
Román GC (2007) Autism: transient in utero hypothyroxinemia related to maternal flavonoid ingestion during pregnancy and to other environmental antithyroid agents. J Neurol Sci 262(1–2):15–26
Ozpinar A, Kelestimur F, Songur Y, Can O, Valentin L, Caldwell K, Arikan E, Unsal I et al (2014) Iodine status in Turkish populations and exposure to iodide uptake inhibitors. PLoS One 9(2):e88206
Rogan WJ, Paulson JA, Baum C, Brock-Utne AC, Brumberg HL, Campbell CC, Lanphear BP, Lowry JA et al (2014) Iodine deficiency, pollutant chemicals, and the thyroid: new information on an old problem. Pediatrics 133:1163–1166
Tonacchera M, Viacava P, Fanelli G, Agretti P, De Marco G, De Servi M, Di Cosmo C, Chiovato L et al (2004) The sodium-iodide symporter protein is always present at a low expression and confined to the cell membrane in nonfunctioning nonadenomatous nodules of toxic nodular goitre. Clin Endocrinol (Oxf) 61(1):40–45
Kirk AB (2006) Environmental perchlorate: why it matters. Anal Chim Acta 567(1):4–12
Lumen A, Mattie DR, Fisher JW (2013) Evaluation of perturbations in serum thyroid hormones during human pregnancy due to dietary iodide and perchlorate exposure using a biologically based dose–response model. Toxicol Sci 133(2):320–341
Melse-Boonstra A, Mackenzie I (2013) Iodine deficiency, thyroid function and hearing deficit: a review. Nutr Res Rev 26(2):110–117
Conde-Agudelo A, Romero R (2009) Antenatal magnesium sulfate for the prevention of cerebral palsy in preterm infants less than 34 weeks’ gestation: a systematic review and metaanalysis. Am J Obstet Gynecol 200(6):595–609
Román GC, Ghassabian A, Bongers-Schokking JJ, Jaddoe VW, Hofman A, de Rijke YB, Verhulst FC, Tiemeier H (2013) Association of gestational maternal hypothyroxinemia and increased autism risk. Ann Neurol 74(5):733–742
Hynes KL, Otahal P, Hay I, Burgess JR (2013) Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the gestational iodine cohort. J Clin Endocrinol Metab 98:1954–1962
Bath SC, Steer CD, Golding J, Emmett P, Rayman MP (2013) Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 382:331–337
Berbel P, Mestre JL, Santamaría A, Palazón I, Franco A, Graells M, González-Torga A, de Escobar GM (2009) Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: the importance of early iodine supplementation. Thyroid 19:511–519
Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, Vulsma T, Wiersinga WM et al (1999) Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 50:149–155
Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL (2003) Maternal hypothyroxinemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 59:282–288
Skeaff SA (2011) Iodine deficency in pregnancy: the effect on neurodevelopment in the child. Nutrients 3:265–273
Costeira MJ, Oliveira P, Santos NC, Ares S, Saenz-Rico B, de Escobar GM, Palha JA (2011) Psychomotor development of children from an iodine-deficient region. J Pediatr 159:447–453
Korevaar TI, Schalekamp-Timmermans S, de Rijke YB, Visser WE, Visser W, de Muinck Keizer-Schrama SM, Hofman A, Ross HA et al (2013) Hypothyroxinemia and TPO-antibody positivity are risk factors for premature delivery: the generation R study. J Clin Endocrinol Metab 98(11):4382–4390
Ares S, Escobar-Morreale HF, Quero J, Durán S, Presas MJ, Herruzo R, Morreale de Escobar G (1997) Neonatal hypothyroxinemia: effects of iodine intake and premature birth. J Clin Endocrinol Metab 82(6):1704–1712
van Wassenaer AG, Kok JH (2004) Hypothyroxinaemia and thyroid function after preterm birth. Semin Neonatol 9(1):3–11
Williams FL, Hume R (2008) Perinatal factors affecting thyroid hormone status in extreme preterm infants. Semin Perinatol 32:398–402
Williams FL, Mires GJ, Barnett C, Ogston SA, van Toor H, Visser TJ, Hume R, Scottish Preterm Thyroid Group (2005) Transient hypothyroxinemia in preterm infants: the role of cord sera thyroid hormone levels adjusted for prenatal and intrapartum factors. J Clin Endocrinol Metab 90:4599–4606
Hong T, Paneth N (2008) Maternal and infant thyroid disorders and cerebral palsy. Semin Perinatol 32(6):438–445
Rovet J, Simic N (2008) The role of transient hypothyroxinemia of prematurity in development of visual abilities. Semin Perinatol 32(6):431–437
Allen MC (2008) Neurodevelopmental outcomes of preterm infants. Curr Opin Neurol 21(2):123–128
Reuss ML, Paneth N, Pinto-Martin JA, Lorenz JM, Susser M (1996) The relation of transient hypothyroxinemia in preterm infants to neurologic development at two years of age. N Engl J Med 334(13):821–827
Hamm MP, Cherry NM, Martin JW, Bamforth F, Burstyn I (2009) The impact of isolated maternal hypothyroxinemia on perinatal morbidity. J Obstet Gynaecol Can 31(11):1015–1021
Storey NM, Gentile S, Ullah H, Russo A, Muessel M, Erxleben C, Armstrong DL (2006) Rapid signaling at the plasma membrane by a nuclear receptor for thyroid hormone. Proc Natl Acad Sci U S A 103:5197–5201
Wiesner RJ, Kurowski TT, Zak R (1992) Regulation by thyroid hormone of nuclear and mitochondrial genes encoding subunits of cytochrome-c oxidase in rat liver and skeletal muscle. Mol Endocrinol 6:1458–1467
Zamoner A, Pessoa-Pureur R (2011) Nongenomic actions of thyroid hormones: every why has a wherefore. Immunol Endocr Metab Agents Med Chem 11:165–178
Berbel P, Navarro D, Román GC (2014) An evo-devo approach to thyroid hormones and cerebral cortex development: etiological implications for autism. Front Endocrinol 5:146. doi:10.3389/fendo.2014.00146
Chatonnet F, Flamant F, Morte B (2014) A temporary compendium of thyroid hormone target genes in brain. Biochim Biophys Acta. doi:10.1016/j.bbagrm.2014.05.023
Morte B, Díez D, Ausó E, Belinchón MM, Gil-Ibáñez P, Grijota-Martínez C, Navarro D, de Escobar GM et al (2010) Thyroid hormone regulation of gene expression in the developing rat fetal cerebral cortex: prominent role of the Ca2+/calmodulin-dependent protein kinase IV pathway. Endocrinology 151:810–820
Siegrist-Kaiser CA, Juge-Aubry C, Tranter MP, Ekenbarger DM, Leonard JL (1990) Thyroxine-dependent modulation of actin polymerization in cultured astrocytes. A novel, extranuclear action of thyroid hormone. J Biol Chem 265:5296–5302
Leonard JL, Farwell AP (1997) TH-regulated actin polymerization in brain. Thyroid 7:147–151
Davis PJ, Shih A, Lin HY, Martino LJ, Davis FB (2000) Thyroxine promotes association of mitogen-activated protein kinase and nuclear thyroid hormone receptor (TR) and causes serine phosphorylation of TR. J Biol Chem 275:38032–38039
Visser WE, Friesema EC, Visser TJ (2011) Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol Endocrinol 25(1):1–14
Schwartz CE, Stevenson RE (2007) The MCT8 thyroid hormone transporter and Allan-Herndon-Dudley syndrome. Best Pract Res Clin Endocrinol Metab 21:307–321
Babu S, Sinha RA, Mohan V, Rao G, Pal A, Pathak A, Singh M, Godbole MM (2011) Effect of hypothyroxinemia on thyroid hormone responsiveness and action during rat postnatal neocortical development. Exp Neurol 228(1):91–98
Chan S, Kilby MD (2000) Thyroid hormone and central nervous system development. J Endocrinol 165(1):1–8
Heuer H, Mason CA (2003) Thyroid hormone induces cerebellar Purkinje cell dendritic development via the thyroid hormone receptor α1. J Neurosci 23(33):10604–10612
Gao FB, Apperly J, Raff M (1998) Cell-intrinsic timers and thyroid hormone regulate the probability of cell-cycle withdrawal and differentiation of oligodendrocyte precursor cells. Dev Biol 197:54–66
Billon N, Tokumoto Y, Forrest D, Raff M (2001) Role of thyroid hormone receptors in timing oligodendrocyte differentiation. Dev Biol 235(1):110–120
Billon N, Jolicoeur C, Tokumoto Y, Vennström B, Raff M (2002) Normal timing of oligodendrocyte development depends on thyroid hormone receptor alpha 1 (TRα1). EMBO J 21(23):6452–6460
Tsunekawa K, Murakami M (2014) Thyroid disease caused by receptor abnormality. Rinsho Byori 62(1):60–66
Shi C, Meng Q, Wood DW (2014) Analysis of the roles of mutations in thyroid hormone receptor-β by a bacterial biosensor system. J Mol Endocrinol 52(1):55–66
Glinoer D (2001) Pregnancy and iodine. Thyroid 11:471–481
Moleti M, Trimarchi F, Vermiglio F (2014) Thyroid physiology in pregnancy. Endocr Pract 20(6):589–596
Patel J, Landers K, Li H, Mortimer RH, Richard K (2011) Delivery of maternal thyroid hormones to the fetus. Trends Endocrinol Metab 22:164–170
Puig-Domingo M, Vila L (2013) The implications of iodine and its supplementation during pregnancy in fetal brain development. Curr Clin Pharmacol 8(2):97–109
Pearce EN (2012) Effects of iodine deficiency in pregnancy. J Trace Elem Med Biol 26:131–133
Moleti M, Trimarchi F, Vermiglio F (2011) Doubts and concerns about isolated maternal hypothyroxinemia. J Thyroid Res 2011:463029
Pedraza PE, Obregon MJ, Escobar-Morreale HF, del Rey FE, de Escobar GM (2006) Mechanisms of adaptation to iodine deficiency in rats: thyroid status is tissue specific. Its relevance for man. Endocrinology 147(5):2098–2108
Sharlin DS, Tighe D, Gilbert ME, Zoeller RT (2008) The balance between oligodendrocyte and astrocyte production in major white matter tracts in linearly related to serum total thyroxine. Endocrinology 149(5):2527–2536
Sinha RA, Pathak A, Mohan V, Bandyopadhyay S, Rastogi L, Godbole MM (2008) Maternal thyroid hormone: a strong repressor of neuronal nitric oxide synthase in rat embryonic neocortex. Endocrinology 149(9):4396–4401
Lavado-Autric R, Ausó E, García-Velasco JV, Arufe Mdel C, Escobar del Rey F, Berbel P, Morreale de Escobar G (2003) Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. J Clin Invest 111:1073–1082
Gilbert ME, Ramos RL, McCloskey DP, Goodman JH (2014) Subcortical band heterotopia in rat offspring following maternal hypothyroxinemia: structural and functional characteristics. J Neuroendocrinol 26(8):528–541
Farwell AP, Dubord-Tomasetti SA, Pietrzykowski AZ, Stachelek SJ, Leonard JL (2005) Regulation of cerebellar neuronal migration and neurite outgrowth by thyroxine and 3,3′,5′-triiodothyronine. Brain Res Dev Brain Res 154(1):121–135
Zamoner A, Funchal C, Jacques-Silva MC, Gottfried C, Barreto Silva FR, Pessoa-Pureur R (2007) Thyroid hormones reorganize the cytoskeleton of glial cells through Gfap phosphorylation and RhoA-dependent mechanisms. Cell Mol Neurobiol 27(7):845–865
Davis PJ, Leonard JL, Davis FB (2008) Mechanisms of nongenomic actions of thyroid hormone. Front Neuroendocrinol 29(2):211–218
Mitchison T, Kirschner M (1988) Cytoskeletal dynamics and nerve growth. Neuron 1(9):761–772
Wang Y, Wei W, Wang Y, Dong J, Song B, Min H, Teng W, Chen J (2013) Neurotoxicity of developmental hypothyroxinemia and hypothyroidism in rats: impairments of long-term potentiation are mediated by phosphatidylinositol 3-kinase signaling pathway. Toxicol Appl Pharmacol 271:257–265
Wang Y, Wei W, Song B, Wang Y, Dong J, Min H, Chen J (2014) Developmental hypothyroxinemia caused by mild iodine deficiency leads to HFS-induced LTD in rat hippocampal CA1 region: involvement of AMPA receptor. Mol Neurobiol 50(2):348–357
Wei W, Wang Y, Dong J, Wang Y, Min H, Song B, Shan Z, Teng W et al (2014) Hypothyroxinemia induced by maternal mild iodine deficiency impairs hippocampal myelinated growth in lactational rats. Environ Toxicol. doi:10.1002/tox.21997
Brooks VB (1984) Cerebellar functions in motor control. Hum Neurobiol 2:251–260
Wang Y, Wang Y, Dong J, Wei W, Song B, Min H, Yu Y, Lei X et al (2014) Developmental hypothyroxinemia and hypothyroidism reduce proliferation of cerebellar granule neuron precursors in rat offspring by downregulation of the sonic hedgehog signaling pathway. Mol Neurobiol 49:1143–1152
Harry GJ, Hooth MJ, Vallant M, Behl M, Travlos GS, Howard JL, Price CJ, McBride S et al (2014) Developmental neurotoxicity of 3,3′,4,4′-tetrachloroazobenzene with thyroxine deficit: sensitivity of glia and dentate granule neurons in the absence of behavioral changes. Toxics 2(3):496–532
Zhang L, Sun YN, Li YM, Lin LX, Ye Y, Yan YQ, Chen ZP (2011) Effect of different iodine nutrition on cerebellum Pcp-2 in rat offspring during lactation. Biol Trace Elem Res 143:1629–1639
Man EB (1972) Thyroid function in pregnancy and infancy. Maternal hypothyroxinemia and retardation of progeny. CRC Crit Rev Clin Lab Sci 3(2):203–225
Trentin AG (2006) Thyroid hormone and astrocyte morphogenesis. J Endocrinol 189(2):189–197
Vila L, Velasco I, González S, Morales F, Sánchez E, Torrejón S, Soldevila B, Stagnaro-Green A et al (2013) Controversies in endocrinology. On the need for universal thyroid screening in pregnant women. Eur J Endocrinol 170(1):R17–R30
Lazarus JH, Bestwick JP, Channon S, Paradice R, Maina A, Rees R, Chiusano E, John R et al (2012) Antenatal thyroid screening and childhood cognitive function. N Engl J Med 366(6):493–501
Trumpff C, De Schepper J, Tafforeau J, Van Oyen H, Vanderfaeillie J, Vandevijvere S (2013) Mild iodine deficiency in pregnancy in Europe and its consequences for cognitive and psychomotor development of children: a review. J Trace Elem Med Biol 27(3):174–183
Zhou SJ, Anderson AJ, Gibson RA, Makrides M (2013) Effect of iodine supplementation in pregnancy on child development and other clinical outcomes: a systematic review of randomized controlled trials. Am J Clin Nutr 98(5):1241–1254
Ferreir SM, Navarro AM, Magalhães PK, Maciel LM (2014) Iodine insufficiency in pregnant women from the State of São Paulo. Arq Bras Endocrinol Metabol 58:282–287
Méndez-Villa L, Elton-Puente JE, Solís-S JC, Sampson-Zaldívar E, García-G C, Villalobos P, Colarossi A, García OP et al (2014) Iodine nutrition and thyroid function assessment in childbearing age women from Queretaro, Mexico. Nutr Hosp 29:204–211
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers 81102126, 30800896), and Important Platform of Science and Technology for the Universities in Liaoning Province (grant number 16010).
Conflict of Interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hui Min and Jing Dong contributed equally to this work.
Rights and permissions
About this article
Cite this article
Min, H., Dong, J., Wang, Y. et al. Maternal Hypothyroxinemia-Induced Neurodevelopmental Impairments in the Progeny. Mol Neurobiol 53, 1613–1624 (2016). https://doi.org/10.1007/s12035-015-9101-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9101-x