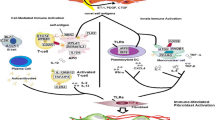
Abstract
The fundamental mechanisms that drive the pathogenesis of systemic sclerosis (SSc) remain elusive, despite over 50 years of investigation. Here, we review recent progress in the understanding of the immunopathogenesis of SSc. In particular, we consider interleukin-13 (IL13), and its upstream and downstream pathways, as an example of an immune system-derived mediator involved in fibrotic and vascular pathology. Emerging results linking pattern-recognition receptors and interferon pathways to SSc are also stressed. We discuss genetic data linking the immune system to SSc risk and efforts to apply animal models to subsets of patients recently resolved by gene expression profiling. These developments will help build a context for better understanding of previous observations and design of the next generation of studies that may eventually lead to effective treatment.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Strehlow D, Korn JH. Biology of the scleroderma fibroblast. Curr Opin Rheumatol. 1998;10(6):572–8.
Sgonc R. The vascular perspective of systemic sclerosis: of chickens, mice and men. Int Arch Allergy Immunol. 1999;120(3):169–76.
Stone OJ. Autoimmunity as a secondary phenomenon in scleroderma (and so-called human adjuvant disease). Med Hypotheses. 1991;34(2):127–30.
Manno R, Boin F. Immunotherapy of systemic sclerosis. Immunotherapy. 2010;2(6):863–78.
Zhou X, Lee JE, Arnett FC, et al. HLA-DPB1 and DPB2 are genetic loci for systemic sclerosis: a genome-wide association study in Koreans with replication in North Americans. Arthritis Rheum. 2009;60(12):3807–14.
•• Radstake TR, Gorlova O, Rueda B, et al. Genome-wide association study of systemic sclerosis identifies CD247 as a new susceptibility locus. Nat Genet. 2010;42(5):426–9. This paper reports the findings of a large GWAS study of SSc, identifying the CD247 locus as involved and confirming previous observations regarding the MHC locus, IRF5, and STAT4.
• Allanore Y, Saad M, Dieude P, et al. Genome-wide scan identifies TNIP1, PSORS1C1, and RHOB as novel risk loci for systemic sclerosis. PLoS Genet. 2011;7(7):e1002091. This GWAS study of SSc confirms the linkage with MHC and identifies the TNIP1, RHOB, and PSORS1C1 loci as involved.
Sharif R, Mayes MD, Tan FK, et al. IRF5 polymorphism predicts prognosis in patients with systemic sclerosis. Ann Rheum Dis. 2012;71(7):1197–202.
Bossini-Castillo L, Martin JE, Broen J, et al. A GWAS follow-up study reveals the association of the IL12RB2 gene with systemic sclerosis in Caucasian populations. Hum Mol Genet. 2012;21(4):926–33.
Bossini-Castillo L, Simeon CP, Beretta L, et al. A multicenter study confirms CD226 gene association with systemic sclerosis-related pulmonary fibrosis. Arthritis Res Ther. 2012;14(2):R85.
Bossini-Castillo L, Broen JC, Simeon CP, et al. A replication study confirms the association of TNFSF4 (OX40L) polymorphisms with systemic sclerosis in a large European cohort. Ann Rheum Dis. 2011;70(4):638–41.
Broen JC, Coenen MJ, Radstake TR. Genetics of systemic sclerosis: an update. Curr Rheumatol Rep. 2012;14(1):11–21.
Romano E, Manetti M, Guiducci S, et al. The genetics of systemic sclerosis: an update. Clin Exp Rheumatol. 2011;29(2 Suppl 65):S75–86.
Beyer C, Schett G, Distler O, Distler JH. Animal models of systemic sclerosis: prospects and limitations. Arthritis Rheum. 2010;62(10):2831–44.
Sargent JL, Whitfield ML. Capturing the heterogeneity in systemic sclerosis with genome-wide expression profiling. Expert Rev Clin Immunol. 2011;7(4):463–73.
LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15(2):202–5.
Milano A, Pendergrass SA, Sargent JL, et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS One. 2008;3(7):e2696.
•• Pendergrass SA, Lemaire R, Francis IP, et al. Intrinsic gene expression subsets of diffuse cutaneous systemic sclerosis are stable in serial skin biopsies. J Invest Dermatol. 2012;132(5):1363–73. This work extends the results of Milano et al., furnishing evidence that the SSc subsets identified are stable and may not simply reflect different stages during the development of SSc.
Yamamoto T. Animal model of systemic sclerosis. J Dermatol. 2010;37(1):26–41.
Artlett CM. Animal models of scleroderma: fresh insights. Curr Opin Rheumatol. 2010;22(6):677–82.
Jaffee BD, Claman HN. Chronic graft-versus-host disease (GVHD) as a model for scleroderma. I. Description of model systems. Cell Immunol. 1983;77(1):1–12.
Fleming JN, Shulman HM, Nash RA, et al. Cutaneous chronic graft-versus-host disease does not have the abnormal endothelial phenotype or vascular rarefaction characteristic of systemic sclerosis. PLoS One. 2009;4(7):e6203.
Ruzek MC, Jha S, Ledbetter S, et al. A modified model of graft-versus-host-induced systemic sclerosis (scleroderma) exhibits all major aspects of the human disease. Arthritis Rheum. 2004;50(4):1319–31.
Kaplan DH, Anderson BE, McNiff JM, et al. Target antigens determine graft-versus-host disease phenotype. J Immunol. 2004;173(9):5467–75.
•• Greenblatt MB, Sargent JL, Farina G, et al. Interspecies comparison of human and murine scleroderma reveals IL-13 and CCL2 as disease subset-specific targets. Am J Pathol. 2012;180(3):1080–94. Here we describe the contribution of IL-13 to the SclGVHD model, including the cellular sources and effects of IL-13. Also, molecular profiling of the SclGVHD model is used to map it to the inflammatory subset of SSc patients.
McCormick LL, Zhang Y, Tootell E, Gilliam AC. Anti-TGF-b treatment prevents skin and lung fibrosis in murine sclerodermatous graft-versus-host disease: a model for human scleroderma. J Immunol. 1999;163:5693–9.
Christmann RB, Wells AU, Capelozzi VL, Silver RM. Gastroesophageal reflux incites interstitial lung disease in systemic sclerosis: clinical, radiologic, histopathologic, and treatment evidence. Semin Arthritis Rheum. 2010;40(3):241–9.
Varga J, Pasche B. Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol. 2009;5(4):200–6.
Abraham D. Connective tissue growth factor: growth factor, matricellular organizer, fibrotic biomarker or molecular target for anti-fibrotic therapy in SSc? Rheumatology (Oxford). 2008;47 Suppl 5:v8–9.
Trojanowska M. Role of PDGF in fibrotic diseases and systemic sclerosis. Rheumatology (Oxford). 2008;47 Suppl 5:v2–4.
Silver RM. Endothelin and scleroderma lung disease. Rheumatology (Oxford). 2008;47 Suppl 5:v25–6.
Minty A, Chalon P, Derocq JM, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362(6417):248–50.
Supajatura V, Ushio H, Nakao A, et al. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest. 2002;109(10):1351–9.
Bellinghausen I, Brand P, Bottcher I, et al. Production of interleukin-13 by human dendritic cells after stimulation with protein allergens is a key factor for induction of T helper 2 cytokines and is associated with activation of signal transducer and activator of transcription-6. Immunology. 2003;108(2):167–76.
Kim EY, Battaile JT, Patel AC, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14(6):633–40.
Barlow JL, McKenzie AN. Nuocytes: expanding the innate cell repertoire in type-2 immunity. J Leukoc Biol. 2011;90(5):867–74.
• Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367–70. With Refs. [38] and [39], this paper identifies nuocytes as a novel IL-13-producing, IL-25 and IL33-responsive, innate immune cell.
• Moro K, Yamada T, Tanabe M, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463(7280):540–4. With Refs. [37] and [39], this paper identifies a novel IL13-producing population that responds to IL-25.
• Price AE, Liang HE, Sullivan BM, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107(25):11489–94. With Refs. [37] and [38], this paper identifies systemically dispersed lineage negative cell that secretes IL-13 in response to IL-25 and IL33.
Nelms K, Keegan AD, Zamorano J, et al. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–38.
Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300(5625):1527–8.
Tabata Y, Khurana Hershey GK. IL-13 receptor isoforms: breaking through the complexity. Curr Allergy Asthma Rep. 2007;7(5):338–45.
Donaldson DD, Whitters MJ, Fitz LJ, et al. The murine IL-13 receptor alpha 2: molecular cloning, characterization, and comparison with murine IL-13 receptor alpha 1. J Immunol. 1998;161(5):2317–24.
Murata T, Husain SR, Mohri H, Puri RK. Two different IL-13 receptor chains are expressed in normal human skin fibroblasts, and IL-4 and IL-13 mediate signal transduction through a common pathway. Int Immunol. 1998;10(8):1103–10.
Daines MO, Tabata Y, Walker BA, et al. Level of expression of IL-13R alpha 2 impacts receptor distribution and IL-13 signaling. J Immunol. 2006;176(12):7495–501.
Wood N, Whitters MJ, Jacobson BA, et al. Enhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor alpha 2. J Exp Med. 2003;197(6):703–9.
Chiaramonte MG, Mentink-Kane M, Jacobson BA, et al. Regulation and function of the interleukin 13 receptor alpha 2 during a T helper cell type 2-dominant immune response. J Exp Med. 2003;197(6):687–701.
Leigh R, Ellis R, Wattie J, et al. Is interleukin-13 critical in maintaining airway hyperresponsiveness in allergen-challenged mice? Am J Respir Crit Care Med. 2004;170(8):851–6.
Fichtner-Feigl S, Young CA, Kitani A et al.: IL-13 signaling via IL-13R alpha2 induces major downstream fibrogenic factors mediating fibrosis in chronic TNBS colitis. Gastroenterology 2008, 135(6):2003–2013, 2013 e2001-2007.
Fichtner-Feigl S, Strober W, Kawakami K, et al. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12(1):99–106.
Hasegawa M, Fujimoto M, Kikuchi K, Takehara K. Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J Rheumatol. 1997;24(2):328–32.
Fuschiotti P, Medsger Jr TA, Morel PA. Effector CD8+ T cells in systemic sclerosis patients produce abnormally high levels of interleukin-13 associated with increased skin fibrosis. Arthritis Rheum. 2009;60(4):1119–28.
Medsger Jr TA, Ivanco DE, Kardava L, et al. GATA-3 up-regulation in CD8+ T cells as a biomarker of immune dysfunction in systemic sclerosis, resulting in excessive interleukin-13 production. Arthritis Rheum. 2011;63(6):1738–47.
Sweiss NJ, Hushaw L, Thenappan T, et al. Diagnosis and management of pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep. 2010;12(1):8–18.
Christmann RB, Hayes E, Pendergrass S, et al. Interferon and alternative activation of monocyte/macrophages in systemic sclerosis-associated pulmonary arterial hypertension. Arthritis Rheum. 2011;63(6):1718–28.
Riccieri V, Rinaldi T, Spadaro A, et al. Interleukin-13 in systemic sclerosis: relationship to nailfold capillaroscopy abnormalities. Clin Rheumatol. 2003;22(2):102–6.
Cutolo M, Sulli A, Smith V. Assessing microvascular changes in systemic sclerosis diagnosis and management. Nat Rev Rheumatol. 2010;6(10):578–87.
Granel B, Allanore Y, Chevillard C, et al. IL13RA2 gene polymorphisms are associated with systemic sclerosis. J Rheumatol. 2006;33(10):2015–9.
Granel B, Chevillard C, Allanore Y, et al. Evaluation of interleukin 13 polymorphisms in systemic sclerosis. Immunogenetics. 2006;58(8):693–9.
Broen JC, Dieude P, Vonk MC, et al. Polymorphisms in the interleukin 4, interleukin 13, and corresponding receptor genes are not associated with systemic sclerosis and do not influence gene expression. J Rheumatol. 2012;39(1):112–8.
Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103(6):779–88.
Zheng T, Oh MH, Oh SY, et al. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. J Invest Dermatol. 2009;129(3):742–51.
Matsushita M, Yamamoto T, Nishioka K. Upregulation of interleukin-13 and its receptor in a murine model of bleomycin-induced scleroderma. Int Arch Allergy Immunol. 2004;135(4):348–56.
Aliprantis AO, Wang J, Fathman JW, et al. Transcription factor T-bet regulates skin sclerosis through its function in innate immunity and via IL-13. Proc Natl Acad Sci USA. 2007;104(8):2827–30.
Belperio JA, Dy M, Burdick MD, et al. Interaction of IL-13 and C10 in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2002;27(4):419–27.
Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83.
Gallina G, Dolcetti L, Serafini P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116(10):2777–90.
Zhu Z, Ma B, Zheng T, et al. IL-13-induced chemokine responses in the lung: role of CCR2 in the pathogenesis of IL-13-induced inflammation and remodeling. J Immunol. 2002;168(6):2953–62.
Palmer G, Gabay C. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol. 2011;7(6):321–9.
Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–90.
Chackerian AA, Oldham ER, Murphy EE, et al. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179(4):2551–5.
Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci USA. 2009;106(22):9021–6.
Lefrancais E, Roga S, Gautier V, et al. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci USA. 2012;109(5):1673–8.
Pichery M, Mirey E, Mercier P, et al. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188(7):3488–95.
Kurowska-Stolarska M, Hueber A, Stolarski B, McInnes IB. Interleukin-33: a novel mediator with a role in distinct disease pathologies. J Intern Med. 2010;269(1):29–35.
Manetti M, Ibba-Manneschi L, Liakouli V, et al. The IL1-like cytokine IL33 and its receptor ST2 are abnormally expressed in the affected skin and visceral organs of patients with systemic sclerosis. Ann Rheum Dis. 2010;69(3):598–605.
Yanaba K, Yoshizaki A, Asano Y, et al. Serum IL-33 levels are raised in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. Clin Rheumatol. 2011;30(6):825–30.
Manetti M, Guiducci S, Ceccarelli C, et al. Increased circulating levels of interleukin 33 in systemic sclerosis correlate with early disease stage and microvascular involvement. Ann Rheum Dis. 2011;70(10):1876–8.
Miller AM. Role of IL-33 in inflammation and disease. J Inflamm (Lond). 2011;8(1):22.
Ho LH, Ohno T, Oboki K, et al. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcepsilonRI signals. J Leukoc Biol. 2007;82(6):1481–90.
Smithgall MD, Comeau MR, Yoon BR, et al. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20(8):1019–30.
Rankin AL, Mumm JB, Murphy E, et al. IL-33 induces IL-13-dependent cutaneous fibrosis. J Immunol. 2009;184(3):1526–35.
Yamamoto T. The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Arch Dermatol Res. 2006;297(8):333–44.
Tan FK, Zhou X, Mayes MD, et al. Signatures of differentially regulated interferon gene expression and vasculotrophism in the peripheral blood cells of systemic sclerosis patients. Rheumatology (Oxford). 2006;45(6):694–702.
York MR, Nagai T, Mangini AJ, et al. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum. 2007;56(3):1010–20.
Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–8.
•• Farina GA, York MR, Di Marzio M, et al. Poly(I:C) drives type I IFN- and TGFbeta-mediated inflammation and dermal fibrosis simulating altered gene expression in systemic sclerosis. J Invest Dermatol. 2010;130(11):2583–93. This paper identifies expression of type I interferon and TGFβ target genes in the skin of SSc patients and demonstrates that chronic subcutaneous infusion of type I interferon eliciting poly I:C stimulates inflammation and fibrosis.
Farina G, York M, Collins C, Lafyatis R. dsRNA activation of endothelin-1 and markers of vascular activation in endothelial cells and fibroblasts. Ann Rheum Dis. 2011;70(3):544–50.
Cavassani KA, Ishii M, Wen H, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205(11):2609–21.
White B. Immunopathogenesis of systemic sclerosis. Rheum Dis Clin North Am. 1996;22(4):695–708.
Roumm AD, Whiteside TL, Medsger Jr TA, Rodnan GP. Lymphocytes in the skin of patients with progressive systemic sclerosis. Quantification, subtyping, and clinical correlations. Arthritis Rheum. 1984;27(6):645–53.
Acknowledgments
Dr Aliprantis is supported by grants from the Burroughs Wellcome Fund and the National Institutes of Health (K08 AR 054859 and R01 AR060363). Dr Aliprantis’ work relevant to SSc was previously supported by the Scleroderma Research Foundation and Sanofi pharmaceuticals. The authors thank Drs Julia Charles, Susan Ritter, and Joerg Ermann for their thoughtful review and discussion of this manuscript.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Scleroderma
Rights and permissions
About this article
Cite this article
Greenblatt, M.B., Aliprantis, A.O. The Immune Pathogenesis of Scleroderma: Context Is Everything. Curr Rheumatol Rep 15, 297 (2013). https://doi.org/10.1007/s11926-012-0297-8
Published:
DOI: https://doi.org/10.1007/s11926-012-0297-8