Abstract
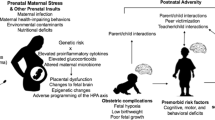
The British epidemiologist Dr. David J. Barker documented the relationship between infant birth weight and later onset of hypertension, coronary heart disease, insulin resistance, and type II diabetes. A stressful in utero environment can cause long-term consequences for offspring through prenatal programming. Prenatal programming most commonly occurs through epigenetic mechanisms and can be dependent on the type and timing of exposure as well as the sex of the fetus. In this review, we highlight the most recent evidence that prenatal programming is implicated in the development of psychiatric disorders in offspring exposed to maternal stress during pregnancy. Methodological differences between studies contribute to unavoidable heterogeneity in study findings. Current data suggest that fetal exposure to maternal hypothalamic-pituitary-adrenal axis dysregulation, excessive glucocorticoids, and inflammation with resulting epigenetic changes at both the placental and fetal levels are important areas of continued investigation.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Barker DJ, Osmond C. Low birth weight and hypertension. BMJ. 1988;297(6641):134–5.
Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2(8663):577–80.
Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301(6761):1111.
Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311(6998):171–4.
Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–7.
Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35(7):595–601.
Gluckman PD, Hanson MA. Maternal constraint of fetal growth and its consequences. Semin Fetal Neonatal Med. 2004;9(5):419–25.
Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20.
Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68(4):314–9. This review summarizes findings from a workshop on Early Life Programming and Neurodevelopmental Disorders held at the University of Pennsylvania in 2009 and reviews the programming effects of nutrition as well as the importance of fetal sex in the outcome of prenatal programming.
Lewis AJ, Galbally M, Gannon T, Symeonides C. Early life programming as a target for prevention of child and adolescent mental disorders. BMC Med. 2014;12:33.
Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology. 2013;38(9):1858–73.
Hansen D, Lou HC, Olsen J. Serious life events and congenital malformations: a national study with complete follow-up. Lancet. 2000;356(9233):875–80.
Zhu JL, Olsen J, Sorensen HT, Li J, Nohr EA, Obel C, et al. Prenatal maternal bereavement and congenital heart defects in offspring: a registry-based study. Pediatrics. 2013;131(4):e1225–30.
Schuurmans C, Kurrasch DM. Neurodevelopmental consequences of maternal distress: what do we really know? Clin Genet. 2013;83(2):108–17.
Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009;3:19.
O’Donnell K, O’Connor TG, Glover V. Prenatal stress and neurodevelopment of the child: focus on the HPA axis and role of the placenta. Dev Neurosci. 2009;31(4):285–92.
Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry. 2001;58(11):1032–7.
Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14.
Brown AS, Vinogradov S, Kremen WS, Poole JH, Deicken RF, Penner JD, et al. Prenatal exposure to maternal infection and executive dysfunction in adult schizophrenia. Am J Psychiatry. 2009;166(6):683–90.
O’Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O’Connor TG, Glover V. Maternal prenatal anxiety and downregulation of placental 11β-HSD2. Psychoneuroendocrinology. 2012;37(6):818–26.
Howerton CL, Morgan CP, Fischer DB, Bale TL. O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development. Proc Natl Acad Sci U S A. 2013;110(13):5169–74.
Susser E, Lin SP, Brown AS, Lumey LH, Erlenmeyer-Kimling L. No relation between risk of schizophrenia and prenatal exposure to influenza in Holland. Am J Psychiatry. 1994;151(6):922–4.
Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, Labovitz D, et al. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 1996;53(1):25–31.
Susser E, St Clair D, He L. Latent effects of prenatal malnutrition on adult health: the example of schizophrenia. Ann N Y Acad Sci. 2008;1136:185–92.
Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol. 2010;90(3):285–326.
Matrisciano F, Tueting P, Dalal I, Kadriu B, Grayson DR, Davis JM, et al. Epigenetic modifications of GABAergic interneurons are associated with the schizophrenia-like phenotype induced by prenatal stress in mice. Neuropharmacology. 2013;68:184–94.
Bock J, Rether K, Groger N, Xie L, Braun K. Perinatal programming of emotional brain circuits: an integrative view from systems to molecules. Front Neurosci. 2014;8:11. Reviews animal models showing that early life stress causes neuronal damage in the prefrontal and limbic brain regions as well data showing that in some cases, early life stress promotes resilience.
Class QA, Khashan AS, Lichtenstein P, Langstrom N, D’Onofrio BM. Maternal stress and infant mortality: the importance of the preconception period. Psychol Sci. 2013;24(7):1309–16. A study with a total of 20,651 offspring which evaluated preconception and prenatal stress (defined as death of a first-degree relative of the mother) on infant mortality. The results suggested that the period immediately before conception may be a sensitive developmental period with ramifications for infant mortality risk.
O’Connor TG, Heron J, Glover V, Alspac Study Team. Antenatal anxiety predicts child behavioral/emotional problems independently of postnatal depression. J Am Acad Child Adolesc Psychiatry. 2002;41(12):1470–7.
Huizink AC, Robles de Medina PG, Mulder EJ, Visser GH, Buitelaar JK. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry. 2003;44(6):810–8.
Bale TL. Sex differences in prenatal epigenetic programming of stress pathways. Stress. 2011;14(4):348–56.
Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav. 2007;91(1):55–65.
Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28(36):9055–65.
Zucchi FC, Yao Y, Ward ID, Ilnytskyy Y, Olson DM, Benzies K, et al. Maternal stress induces epigenetic signatures of psychiatric and neurological diseases in the offspring. PLoS One. 2013;8(2):e56967.
Bronson SL, Bale TL. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology. 2014;155(7):2635–46.
Garcia-Caceres C, Lagunas N, Calmarza-Font I, Azcoitia I, Diz-Chaves Y, Garcia-Segura LM, et al. Gender differences in the long-term effects of chronic prenatal stress on the HPA axis and hypothalamic structure in rats. Psychoneuroendocrinology. 2010;35(10):1525–35.
Spauwen J, Krabbendam L, Lieb R, Wittchen HU, van Os J. Early maternal stress and health behaviours and offspring expression of psychosis in adolescence. Acta Psychiatr Scand. 2004;110(5):356–64.
Rodriguez A, Bohlin G. Are maternal smoking and stress during pregnancy related to ADHD symptoms in children? J Child Psychol Psychiatry. 2005;46(3):246–54.
Costello EJ, Worthman C, Erkanli A, Angold A. Prediction from low birth weight to female adolescent depression: a test of competing hypotheses. Arch Gen Psychiatry. 2007;64(3):338–44.
Grizenko N, Fortier ME, Zadorozny C, Thakur G, Schmitz N, Duval R, et al. Maternal stress during pregnancy, ADHD symptomatology in children and genotype: gene-environment interaction. J Can Acad Child Adolesc Psychiatry. 2012;21(1):9–15.
Glover V, Hill J. Sex differences in the programming effects of prenatal stress on psychopathology and stress responses: an evolutionary perspective. Physiol Behav. 2012;106(5):736–40.
Van den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2008;33(3):536–45.
McGrath J, Saha S, Welham J, El Saadi O, MacCauley C, Chant D. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med. 2004;2:13.
Schmitt A, Malchow B, Hasan A, Falkai P. The impact of environmental factors in severe psychiatric disorders. Front Neurosci. 2014;8:19.
Goldstein JM, Seidman LJ, O’Brien LM, Horton NJ, Kennedy DN, Makris N, et al. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Arch Gen Psychiatry. 2002;59(2):154–64.
Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, et al. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 2008;65(2):146–52.
Class QA, Abel KM, Khashan AS, Rickert ME, Dalman C, Larsson H, et al. Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychol Med. 2014;44(1):71–84.
Dorrington S, Zammit S, Asher L, Evans J, Heron J, Lewis G. Perinatal maternal life events and psychotic experiences in children at twelve years in a birth cohort study. Schizophr Res. 2014;152(1):158–63.
Huttunen MO, Niskanen P. Prenatal loss of father and psychiatric disorders. Arch Gen Psychiatry. 1978;35(4):429–31.
Myhrman A, Rantakallio P, Isohanni M, Jones P, Partanen U. Unwantedness of a pregnancy and schizophrenia in the child. Br J Psychiatry. 1996;169(5):637–40.
Sacker A, Done DJ, Crow TJ, Golding J. Antecedents of schizophrenia and affective illness. Obstetric complications. Br J Psychiatry. 1995;166(6):734–41.
Selten JP, van der Graaf Y, van Duursen R, Gispen-de Wied CC, Kahn RS. Psychotic illness after prenatal exposure to the 1953 Dutch flood disaster. Schizophr Res. 1999;35(3):243–5.
Selten JP, Cantor-Graae E, Nahon D, Levav I, Aleman A, Kahn RS. No relationship between risk of schizophrenia and prenatal exposure to stress during the Six-Day War or Yom Kippur War in Israel. Schizophr Res. 2003;63(1–2):131–5.
Cannon TD, Rosso IM, Hollister JM, Bearden CE, Sanchez LE, Hadley T. A prospective cohort study of genetic and perinatal influences in the etiology of schizophrenia. Schizophr Bull. 2000;26(2):351–66.
Zornberg GL, Buka SL, Tsuang MT. Hypoxic-ischemia-related fetal/neonatal complications and risk of schizophrenia and other nonaffective psychoses: a 19-year longitudinal study. Am J Psychiatry. 2000;157(2):196–202.
Zornberg GL, Buka SL, Tsuang MT. The problem of obstetrical complications and schizophrenia. Schizophr Bull. 2000;26(2):249–56.
van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468(7321):203–12.
Holloway T, Moreno JL, Umali A, Rayannavar V, Hodes GE, Russo SJ, et al. Prenatal stress induces schizophrenia-like alterations of serotonin 2A and metabotropic glutamate 2 receptors in the adult offspring: role of maternal immune system. J Neurosci. 2013;33(3):1088–98.
Baron-Cohen S, Scott FJ, Allison C, Williams J, Bolton P, Matthews FE, et al. Prevalence of autism-spectrum conditions: UK school-based population study. Br J Psychiatry. 2009;194(6):500–9.
Depino AM. Peripheral and central inflammation in autism spectrum disorders. Mol Cell Neurosci. 2013;53:69–76.
Ciaranello AL, Ciaranello RD. The neurobiology of infantile autism. Annu Rev Neurosci. 1995;18:101–28.
Singh VK, Jensen RL. Elevated levels of measles antibodies in children with autism. Pediatr Neurol. 2003;28(4):292–4.
Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40(12):1423–30.
Zerbo O, Qian Y, Yoshida C, Grether JK, Van de Water J, Croen LA. Maternal infection during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2013 Dec 24.
Atladottir HO, Thorsen P, Schendel DE, Ostergaard L, Lemcke S, Parner ET. Association of hospitalization for infection in childhood with diagnosis of autism spectrum disorders: a Danish cohort study. Arch Pediatr Adolesc Med. 2010;164(5):470–7.
Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17(4):448–59.
Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26(4):607–16.
Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491–7.
Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129(5):e1121–8.
Reynolds LC, Inder TE, Neil JJ, Pineda RG, Rogers CE. Maternal obesity and increased risk for autism and developmental delay among very preterm infants. J Perinatol. 2014;34:688–92.
Dodds L, Fell DB, Shea S, Armson BA, Allen AC, Bryson S. The role of prenatal, obstetric and neonatal factors in the development of autism. J Autism Dev Disord. 2011;41(7):891–902.
Sullivan EL, Nousen EK, Chamlou KA. Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiol Behav. 2014;123:236–42.
Ashwood P, Kwong C, Hansen R, Hertz-Picciotto I, Croen L, Krakowiak P, et al. Brief report: plasma leptin levels are elevated in autism: association with early onset phenotype? J Autism Dev Disord. 2008;38(1):169–75.
Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26(3):383–92.
Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17(11–12):953–66.
Froehlich-Santino W, Londono Tobon A, Cleveland S, Torres A, Phillips J, Cohen B, et al. Prenatal and perinatal risk factors in a twin study of autism spectrum disorders. J Psychiatr Res. 2014;54:100–8.
Zimmerman AW, Connors SL. Neuroscience. Could autism be treated prenatally? Science. 2014;343(6171):620–1.
Gaynes BN, Christian R, Saavedra LM, Wines R, Jonas DE, Viswanathan M, et al. Attention-deficit/hyperactivity disorder: identifying high priority future research needs. J Psychiatr Pract. 2014;20(2):104–17.
Li J, Olsen J, Vestergaard M, Obel C. Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: a nationwide follow-up study in Denmark. Eur Child Adolesc Psychiatry. 2010;19(10):747–53.
Lee CY, Chang YY, Lung FW. The marriage-related risk factors during maternal pregnancy in children with attention-deficit hyperactivity disorder. Child Care Health Dev. 2006;32(2):205–11.
Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev. 2004;75(4):1085–97.
Martini J, Knappe S, Beesdo-Baum K, Lieb R, Wittchen HU. Anxiety disorders before birth and self-perceived distress during pregnancy: associations with maternal depression and obstetric, neonatal and early childhood outcomes. Early Hum Dev. 2010;86(5):305–10.
French NP, Hagan R, Evans SF, Mullan A, Newnham JP. Repeated antenatal corticosteroids: effects on cerebral palsy and childhood behavior. Am J Obstet Gynecol. 2004;190(3):588–95.
Gender and women’s mental health [Internet]. Available from: http://www.who.int/mental_health/prevention/genderwomen/en/.
Fisher D, Baird J, Payne L, Lucas P, Kleijnen J, Roberts H, et al. Are infant size and growth related to burden of disease in adulthood? A systematic review of literature. Int J Epidemiol. 2006;35(5):1196–210.
Vasiliadis HM, Gilman SE, Buka SL. Fetal growth restriction and the development of major depression. Acta Psychiatr Scand. 2008;117(4):306–12.
Alati R, Lawlor DA, Mamun AA, Williams GM, Najman JM, O’Callaghan M, et al. Is there a fetal origin of depression? Evidence from the mater university study of pregnancy and its outcomes. Am J Epidemiol. 2007;165(5):575–82.
Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: a developmental model for understanding mechanisms of transmission. Psychol Rev. 1999;106(3):458–90.
Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19(4):296–308.
Pearson RM, Evans J, Kounali D, Lewis G, Heron J, Ramchandani PG, et al. Maternal depression during pregnancy and the postnatal period: risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry. 2013;70(12):1312–9.
Betts KS, Williams GM, Najman JM, Alati R. The relationship between maternal depressive, anxious, and stress symptoms during pregnancy and adult offspring behavioral and emotional problems. Depress Anxiety. 2014 Apr 30.
Darnaudery M, Maccari S. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev. 2008;57(2):571–85.
Blakeley PM, Capron LE, Jensen AB, O’Donnell KJ, Glover V. Maternal prenatal symptoms of depression and down regulation of placental monoamine oxidase A expression. J Psychosom Res. 2013;75(4):341–5.
O’Donnell KJ, Glover V, Barker ED, O’Connor TG. The persisting effect of maternal mood in pregnancy on childhood psychopathology. Dev Psychopathol. 2014;26(2):393–403. Most recent data from the Avon Longitudinal Study of Parents and Children cohort, a prospective, longitudinal study of a large community sample (n = 7944). Severe prenatal anxiety was associated with a twofold increase in the risk of a childhood mental disorder (12.31 versus 6.83 %) compared to low-anxiety mothers. The effects did not decrease as the child aged into adolescence.
O’Donnell KJ, Glover V, Jenkins J, Browne D, Ben-Shlomo Y, Golding J, et al. Prenatal maternal mood is associated with altered diurnal cortisol in adolescence. Psychoneuroendocrinology. 2013;38(9):1630–8.
Walker AK, Nakamura T, Byrne RJ, Naicker S, Tynan RJ, Hunter M, et al. Neonatal lipopolysaccharide and adult stress exposure predisposes rats to anxiety-like behaviour and blunted corticosterone responses: implications for the double-hit hypothesis. Psychoneuroendocrinology. 2009;34(10):1515–25.
Sominsky L, Fuller EA, Bondarenko E, Ong LK, Averell L, Nalivaiko E, et al. Functional programming of the autonomic nervous system by early life immune exposure: implications for anxiety. PLoS One. 2013;8(3):e57700.
Walker AK, Nakamura T, Hodgson DM. Neonatal lipopolysaccharide exposure alters central cytokine responses to stress in adulthood in Wistar rats. Stress. 2010;13(6):506–15.
Sasaki A, de Vega WC, St-Cyr S, Pan P, McGowan PO. Perinatal high fat diet alters glucocorticoid signaling and anxiety behavior in adulthood. Neuroscience. 2013;240:1–12.
Acknowledgments
This paper is supported by funding from NIMH grant MH09910 (P.I. Epperson and Bale), grant NIMH K23 MH092399 (P.I. Kim) and grants MH073030, MH087597, and MH091258 (P.I. Bale).
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Deborah R. Kim declares that she has no conflict of interest.
Tracy L. Bale declares that she has no conflict of interest.
C. Neill Epperson has received consultancy fees and paid travel accommodations from UmeCrine Mood, expert testimony fees from Forrest, and grants from Shire and has stock options in Pfizer, Merck, AbbVie, Abbot, and Johnson & Johnson.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Women’s Mental Health
Rights and permissions
About this article
Cite this article
Kim, D.R., Bale, T.L. & Epperson, C.N. Prenatal Programming of Mental Illness: Current Understanding of Relationship and Mechanisms. Curr Psychiatry Rep 17, 5 (2015). https://doi.org/10.1007/s11920-014-0546-9
Published:
DOI: https://doi.org/10.1007/s11920-014-0546-9