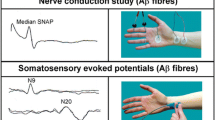
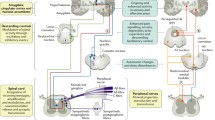
Abstract
Quantitative sensory testing (QST) is a widely accepted tool to investigate somatosensory changes in pain patients. Many different protocols have been developed in clinical pain research within recent years. In this review, we provide an overview of QST and tested neuroanatomical pathways, including peripheral and central structures. Based on research studies using animal and human surrogate models of neuropathic pain, possible underlying mechanisms of chronic pain are discussed. Clinically, QST may be useful for 1) the identification of subgroups of patients with different underlying pain mechanisms; 2) prediction of therapeutic outcomes; and 3) quantification of therapeutic interventions in pain therapy. Combined with sensory mapping, QST may provide useful information on the site of neural damage and on mechanisms of positive and negative somatosensory abnormalities. The use of QST in individual patients for diagnostic purposes leading to individualized therapy is an interesting concept, but needs further validation.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Cruccu G, Aminoff MJ, Curio G, et al. Recommendations for the clinical use of somatosensory-evoked potentials. Clin Neurophysiol. 2008;119:1705–19. This guideline by the International Federation of Clinical Neurophysiology (IFCN) provides standards for objective tests for the assessment of the somatosensory system.
Treede RD: Das somatosensorische System, in Physiologie des Menschen, Schmidt RF and F. L, Editors. 2007, Springer: Heidelberg. 297–323.
•• Haanpaa M, Attal N, Backonja M, et al.: NeuPSIG guidelines on neuropathic pain assessment. Pain 2011:152:14–27. This review includes recent recommendations and guidelines for the assessment of neuropathic pain, comparing QST with clinical examination, questionnaires, electrophysiology, and skin biopsy.
• Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–5. A new definition and a grading system of Neuropathic Pain are presented in this paper, emphasising the relevance of sensory signs in diagnosis.
• Shy ME, Frohman EM, So YT, et al. Quantitative sensory testing: report of the therapeutics and technology assessment subcommittee of the American academy of neurology. Neurology. 2003;60:898–904. This paper presents the use of QST in diagnosis of peripheral neuropathies, especially in small fiber neuropathies.
• Magerl W, Krumova EK, Baron R, et al.: Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain 2010:151:598–605. This paper presents reference data and statistical testing methods for the QST protocol developed by the DFNS.
Gruener G, Dyck PJ. Quantitative sensory testing: methodology, applications, and future directions. J Clin Neurophysiol. 1994;11:568–83.
O'Brien PC, Dyck PJ. Procedures for setting normal values. Neurology. 1995;45:17–23.
•• Rolke R, Magerl W, Campbell KA, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10:77–88. This paper reports on the rationale and the methods of a widespread QST protocol developed by the German Research Network on Neuropathic Pain (DFNS).
Geber C, Klein T, Azad S, et al. Test-retest and interobserver reliability of quantitative sensory testing according to the protocol of the German Research Network on Neuropathic Pain (DFNS): a multi-centre study. Pain. 2011;152:548–56.
• Pigg M, Baad-Hansen L, Svensson P, et al.: Reliability of intraoral quantitative sensory testing (QST). Pain 2010:148:220–6. An intraoral version of the QST protocol according to the DFNS is presented in this paper.
Geber C, Scherens A, Pfau D, et al. Procedure for certification of QST laboratories. Schmerz. 2009;23:65–9.
• Neziri AY, Curatolo M, Nuesch E, et al.: Factor analysis of responses to thermal, electrical, and mechanical painful stimuli supports the importance of multi-modal pain assessment. Pain 2011:152:1146–55. This paper refers to the importance of multimodal testing as sensory testing assesses statistically independent the function of the nociceptive system.
Rader AJ. Surgical decompression in lower-extremity diabetic peripheral neuropathy. J Am Podiatr Med Assoc. 2005;95:446–50.
Bansal V, Kalita J, Misra UK. Diabetic neuropathy. Postgrad Med J. 2006;82:95–100.
Cruccu G, Sommer C, Anand P, et al. EFNS guidelines on neuropathic pain assessment: revised 2009. Eur J Neurol. 2010;17:1010–8.
Yarnitsky D. Quantitative sensory testing. Muscle Nerve. 1997;20:198–204.
Fruhstorfer H, Lindblom U, Schmidt WC. Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiatry. 1976;39:1071–5.
• Dyck PJ, O'Brien PC, Kosanke JL, et al. A 4, 2, and 1 stepping algorithm for quick and accurate estimation of cutaneous sensation threshold. Neurology. 1993;43:1508–12. This paper compares speed and accuracy of several paradigms for threshold estimation and provides information on testing procedures and backgrounds of an earlier highly developed QST protocol (case IV system).
Scherens A, Maier C, Haussleiter IS, et al. Painful or painless lower limb dysesthesias are highly predictive of peripheral neuropathy: comparison of different diagnostic modalities. Eur J Pain. 2009;13:711–8.
Tamburin S, Cacciatori C, Praitano ML, et al. Median nerve small- and large-fiber damage in carpal tunnel syndrome: a quantitative sensory testing study. J Pain. 2011;12:205–12.
• Hansson P, Backonja M, Bouhassira D. Usefulness and limitations of quantitative sensory testing: clinical and research application in neuropathic pain states. Pain. 2007;129:256–9. This review provides an overview over QST procedures compared with bedside tests and focuses on clinical application possibilities.
•• Arendt-Nielsen L, Yarnitsky D: Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain 2009:10:556–72. This review reports on QST methodology in different tissues, including skin, muscles, and viscera.
Jaaskelainen SK, Teerijoki-Oksa T, Forssell H. Neurophysiologic and quantitative sensory testing in the diagnosis of trigeminal neuropathy and neuropathic pain. Pain. 2005;117:349–57.
Geber C, Baumgartner U, Fechir M, et al. Comparison of LEP and QST and their contribution to standard sensory diagnostic assessment of spinal lesions: a pilot study. Neurol Sci. 2011;32:401–10.
• Blankenburg M, Boekens H, Hechler T, et al.: Reference values for quantitative sensory testing in children and adolescents: developmental and gender differences of somatosensory perception. Pain 2010:149:76–88. This study demonstrates QST and presents normative values in children.
Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123:231–43.
Backonja MM, Walk D, Edwards RR, et al. Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain. 2009;25:641–7.
Ziegler EA, Magerl W, Meyer RA, et al. Secondary hyperalgesia to punctate mechanical stimuli. Central sensitization to A-fibre nociceptor input. Brain. 1999;122(Pt 12):2245–57.
Magerl W, Fuchs PN, Meyer RA, et al. Roles of capsaicin-insensitive nociceptors in cutaneous pain and secondary hyperalgesia. Brain. 2001;124:1754–64.
Fruhstorfer H. Thermal sensibility changes during ischemic nerve block. Pain. 1984;20:355–61.
Treede RD, Meyer RA, Raja SN, et al. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421.
Meyer RA, Campbell JN. Myelinated nociceptive afferents account for the hyperalgesia that follows a burn to the hand. Science. 1981;213:1527–9.
LaMotte RH, Thalhammer JG, Robinson CJ. Peripheral neural correlates of magnitude of cutaneous pain and hyperalgesia: a comparison of neural events in monkey with sensory judgments in human. J Neurophysiol. 1983;50:1–26.
Rolke R, Andrews Campbell K, Magerl W, et al. Deep pain thresholds in the distal limbs of healthy human subjects. Eur J Pain. 2005;9:39–48.
• Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–64. This review gives an overview over pathophysiological mechanisms of neuropathic pain and therapeutic targets and argues for mechanism-based rather than diagnosisbased classification of neuropathic pain.
• Woolf CJ: Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011:152:S2-15. This review reports on mechanisms of central sensitization and other mechanisms of persistent pain.
Yarnitsky D, Ochoa JL. Release of cold-induced burning pain by block of cold-specific afferent input. Brain. 1990;113(Pt 4):893–902.
Susser E, Sprecher E, Yarnitsky D. Paradoxical heat sensation in healthy subjects: peripherally conducted by A delta or C fibres? Brain. 1999;122(Pt 2):239–46.
Hansen C, Hopf HC, Treede RD. Paradoxical heat sensation in patients with multiple sclerosis. Evidence for a supraspinal integration of temperature sensation. Brain. 1996;119(Pt 5):1729–36.
Torebjork HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol. 1992;448:765–80.
Treede RD. Hyperalgesia and allodynia: taxonomy, assessment, and mechansims. In: Brune K, Handwerker HO, editors. Hyperalgesia: molecular mechanisms and clincal implications. Seattle: IASP; 2004.
Simone DA, Sorkin LS, Oh U, et al. Neurogenic hyperalgesia: central neural correlates in responses of spinothalamic tract neurons. J Neurophysiol. 1991;66:228–46.
LaMotte RH, Shain CN, Simone DA, et al. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66:190–211.
Blumenstiel K, Gerhardt A, Rolke R, et al. Quantitative sensory testing profiles in chronic back pain are distinct from those in fibromyalgia. Clin J Pain. 2011;27:682–90.
Devor M, Wall PD, Catalan N. Systemic lidocaine silences ectopic neuroma and DRG discharge without blocking nerve conduction. Pain. 1992;48:261–8.
• Maier C, Baron R, Tolle TR, et al.: Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain 2010:150:439–50. QST profiles across different neuropathic pain diagnoses reveal a combination of signs of sensory gain and loss in most individuals.
• Scholz J, Mannion RJ, Hord DE, et al.: A novel tool for the assessment of pain: validation in low back pain. PLoS Med 2009:6:e1000047. This paper gives evidence of the interpretation of QST and other tests as predictive tool for low back pain.
Jensen TS, Baron R. Translation of symptoms and signs into mechanisms in neuropathic pain. Pain. 2003;102:1–8.
• Serra J: Sensory profiles: the cliche and the challenge. Pain 2010:150:384–5. This commentary represents a critical statement on the future role of QST in clinical testing.
Aasvang EK, Brandsborg B, Jensen TS, et al. Heterogeneous sensory processing in persistent postherniotomy pain. Paint. 2010;150:237–42.
Jensen TS, Hansson PT. Chapter 34 Classification of neuropathic pain syndromes based on symptoms and signs. Handb Clin Neurol. 2006;81:517–26.
Geber C, Magerl W, Fondel R, et al. Numbness in clinical and experimental pain–a cross-sectional study exploring the mechanisms of reduced tactile function. Pain. 2008;139:73–81.
Eberle T, Doganci B, Kramer HH, et al. Warm and cold complex regional pain syndromes: differences beyond skin temperature? Neurology. 2009;72:505–12.
Bachmann CG, Rolke R, Scheidt U, et al. Thermal hypoaesthesia differentiates secondary restless legs syndrome associated with small fibre neuropathy from primary restless legs syndrome. Brain. 2010;133:762–70.
Pfau DB, Rolke R, Nickel R, et al. Somatosensory profiles in subgroups of patients with myogenic temporomandibular disorders and Fibromyalgia Syndrome. Pain. 2009;147:72–83.
Lang PM, Schober GM, Rolke R, et al. Sensory neuropathy and signs of central sensitization in patients with peripheral arterial disease. Pain. 2006;124:190–200.
Aasvang EK, Gmaehle E, Hansen JB, et al. Predictive risk factors for persistent postherniotomy pain. Anesthesiology. 2010;112:957–69.
Said-Yekta S, Smeets R, Esteves-Oliveira M, et al.: Verification of Nerve Integrity After Surgical Intervention Using Quantitative Sensory Testing. J Oral Maxillofac Surg 2011.
Attal N, Bouhassira D, Gautron M, et al. Thermal hyperalgesia as a marker of oxaliplatin neurotoxicity: a prospective quantified sensory assessment study. Pain. 2009;144:245–52.
Ducreux D, Attal N, Parker F, et al. Mechanisms of central neuropathic pain: a combined psychophysical and fMRI study in syringomyelia. Brain. 2006;129:963–76.
Eisenberg E, Backonja MM, Fillingim RB, et al. Quantitative sensory testing for spinal cord stimulation in patients with chronic neuropathic pain. Pain Pract. 2006;6:161–5.
Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation (CPM) predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012. doi:10.1016/j.pain.2012.02.021.
Cruccu G, Anand P, Attal N, et al. EFNS guidelines on neuropathic pain assessment. Eur J Neurol. 2004;11:153–62.
Stiasny-Kolster K, Magerl W, Oertel WH, et al. Static mechanical hyperalgesia without dynamic tactile allodynia in patients with restless legs syndrome. Brain. 2004;127:773–82.
Finnerup NB, Biering-Sorensen F, Johannesen IL, et al. Intravenous lidocaine relieves spinal cord injury pain: a randomized controlled trial. Anesthesiology. 2005;102:1023–30.
Gormsen L, Finnerup NB, Almqvist PM, et al. The efficacy of the AMPA receptor antagonist NS1209 and lidocaine in nerve injury pain: a randomized, double-blind, placebo-controlled, three-way crossover study. Anesth Analg. 2009;108:1311–9.
Yucel A, Ozyalcin S, Koknel Talu G, et al. The effect of venlafaxine on ongoing and experimentally induced pain in neuropathic pain patients: a double blind, placebo controlled study. Eur J Pain. 2005;9:407–16.
Walk D. Role of skin biopsy in the diagnosis of peripheral neuropathic pain. Curr Pain Headache Rep. 2009;13:191–6.
Siao P, Cros DP. Quantitative sensory testing. Phys Med Rehabil Clin N Am. 2003;14:261–86.
Arezzo J, Bolton C, Boulton A, et al. Quantitative sensory testing: a consensus report from the Peripheral Neuropathy Association. Neurology. 1993;43:1050–2.
• Dyck PJ, Kennedy WR, Kesserwani H, et al. Limitations of quantitative sensory testing when patients are biased toward a bad outcome. Neurology. 1998;50:1213. This review reports on limitations of QST.
Leffler AS, Hansson P. Painful traumatic peripheral partial nerve injury-sensory dysfunction profiles comparing outcomes of bedside examination and quantitative sensory testing. Eur J Pain. 2008;12:397–402.
Woolf CJ, Bennett GJ, Doherty M, et al. Towards a mechanism-based classification of pain? Pain. 1998;77:227–9.
Geber C, Birklein F. Dissecting post-herniotomy pain–scratching the surface? Pain. 2010;150:215–6.
Treede RD: Funktionsprüfung der nozizeptiven Bahnen durch SEP nach schmerzhaften Laser-Hitzereizen, in Evozierte Potenziale, Hess CW, et al., Editors. 2005, Springer: Heidelberg.599-621.
Disclosures
The authors work in a quantitative sensory testing training center of the German Research Network on Neuropathic Pain, receiving payment for training sessions.
Dr. Doreen B. Pfau has received honoraria and travel expense compensation from Pfizer. C. Geber: none. Dr. Frank Birklein has served on the boards of Eli Lilly & Co., Pfizer, and Astellas Pharma; has received grants from Eli Lilly & Co.; has received honoraria from Eli Lilly & Co., Pfizer, Shire Pharmaceuticals, and Grünenthal GmbH; and has received travel expense compensation from Eli Lilly & Co. and Pfizer. Dr. Rolf-Detlef Treede has served as a consultant for Astellas Pharma, Boehringer Ingelheim, Galderma, Grünenthal GmbH, Eli Lilly & Co., Dr. Kade Merz, Mundipharma, Nycomed, and Pfizer; has been issued patents on behalf of Merz; and has received travel expense compensation from Boehringer Ingelheim, Grünenthal GmbH, Mundipharma, and Pfizer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pfau, D.B., Geber, C., Birklein, F. et al. Quantitative Sensory Testing of Neuropathic Pain Patients: Potential Mechanistic and Therapeutic Implications. Curr Pain Headache Rep 16, 199–206 (2012). https://doi.org/10.1007/s11916-012-0261-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11916-012-0261-3