Abstract
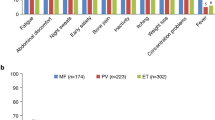
BCR-ABL-negative myeloproliferative neoplasms include primary myelofibrosis, polycythemia vera, and essential thrombocythemia. Clonal stem cell proliferation and dysregulated JAK/STAT molecular pathways characterize these hematologic malignancies. Symptoms experienced by patients are heterogeneous including excessive and disabling fatigue, early satiety, anorexia, pruritus, bone pain, night sweats, cachexia, abdominal pain and discomfort, and cognitive complaints. Patients also experience impaired quality of life along with decreased overall survival. New targeted drug therapies, including JAK2 inhibitors, have demonstrated remarkable success in alleviating the myeloproliferative neoplasm (MPN) symptomatic burden, reducing splenomegaly and improving quality of life while offering overall survival benefit. Within the USA, FDA approval has only been granted to use JAK2 inhibitors in intermediate- to high-risk myelofibrosis. However, given that low-prognostic-scoring patients have been shown to have considerable symptomology, there is a possibility that lower-risk patients may benefit from therapy. More than ever, the need for accurate MPN symptom burden assessment and subsequent addition of targeted therapies is critical in the treatment of MPNs. This article discusses the role of MPN symptom burden and quality of life as therapeutic targets in the context of recent MPN clinical advances.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6:372–5.
Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27:1874–81.
Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22:14–22.
Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007;109:68–76.
Rumi E, Pietra D, Pascutto C, et al. Clinical effect of driver mutations of JAK2, CALR, or MPL in primary myelofibrosis. Blood. 2014;124:1062–9.
Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118:401–8.
Koch CA, Li CY, Mesa RA, Tefferi A. Nonhepatosplenic extramedullary hematopoiesis: associated diseases, pathology, clinical course, and treatment. Mayo Clin Proc. 2003;78:1223–33.
Jackson N, Burt D, Crocker J, Boughton B. Skin mast cells in polycythaemia vera: relationship to the pathogenesis and treatment of pruritus. Br J Dermatol. 1987;116:21–9.
Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–901.
Passamonti F, Rumi E, Caramella M, et al. A dynamic prognostic model to predict survival in post-polycythemia vera myelofibrosis. Blood. 2008;111:3383–7.
Scherber RM, Senyak Z, Dueck A, et al. The MPN fatigue project stage II: understanding the role of comorbid conditions and fatigue-alleviating strategies in MPN fatigue. Haematologica. 2014;S1:a3802.
Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34:13–9.
Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–96.
Tefferi A, Cervantes F, Mesa R, et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood. 2013;122:1395–8.
Mesa RA, Schwager S, Radia D, et al. The Myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res. 2009;33:1199–203.
Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121:4778–81.
Emanuel R, Dueck A, Geyer H, et al. Myeloproliferative (MPN) symptom burden response thresholds: assessment of MPN-SAF TSS quartiles as potential markers of symptom response. Blood. 2013;122:a4067.
Cervantes F, Barosi G, Demory JL, et al. Myelofibrosis with myeloid metaplasia in young individuals: disease characteristics, prognostic factors and identification of risk groups. Br J Haematol. 1998;102:684–90.
Tefferi A, Elliott M. Thrombosis in myeloproliferative disorders: prevalence, prognostic factors, and the role of leukocytes and JAK2V617F. Semin Thromb Hemost. 2007;33:313–20.
Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29:392–7.
Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112:3065–72.
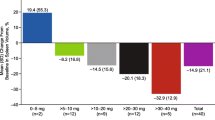
Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–98. This study was one of the initial landmark articles to highlight the benefits of JAK2 inhibitor therapy. Specifically, this trial compared ruxolitinib to best available, standard therapy.
Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. This study was one of the initial landmark articles to highlight the benefits of JAK2 inhibitor therapy. Individuals in this double blind trial were randomized to received either placebo or ruxolitinib.
Verstovsek S, Mesa RA, Gotlib J, et al. Long-term outcome of ruxolitinib treatment in patients with myelofibrosis: durable reductions in spleen volume, improvements in quality of life, and overall survival advantage in COMFORT-I. Blood. 2012;120:1202–9.
Verstovsek S, Passamonti F, Rambaldi A, et al. A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 Inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer. 2014;120:513–20.
Verstovsek S, Passamonti F, Rambaldi A, et al. A phase 2 study of INCB018424, an oral, selective JAK1/JAK2 inhibitor, in patients with advanced polycythemia Vera (PV) and essential thrombocythemia (ET) refractory to hydroxyurea. ASH Ann Meet Abstr. 2009;114:311.
Pardanani AD, Caramazza D, George G, Lasho L, Hogan W, Litzow M, et al. Safety and efficacy of CYT387, a JAK-1/2 inhibitor, for the treatment of myelofibrosis. J Clin Onc. 2011;29;(suppl; abstr 6514).
Verstovsek S, Dean JP, Cernohous P, et al. Pacritinib, a dual JAK2/FLT3 inhibitor: an integrated efficacy and safety analysis of phase II trial data in patients with primary and secondary myelofibrosis (MF) and platelet counts ≤100,000/μl. Blood. 2013;122:395.
Guglielmelli P, Barosi G, Rambaldi A, et al. Safety and efficacy of everolimus, a mTOR inhibitor, as single agent in a phase 1/2 study in patients with myelofibrosis. Blood. 2011;118:2069–76.
Rambaldi A, Dellacasa CM, Finazzi G, et al. A pilot study of the Histone-Deacetylase inhibitor Givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br J Haematol. 2010;150:446–55.
DeAngelo DJ, Mesa RA, Fiskus W, et al. Phase II trial of panobinostat, an oral pan-deacetylase inhibitor in patients with primary myelofibrosis, post-essential thrombocythaemia, and post-polycythaemia vera myelofibrosis. Br J Haematol. 2013;162:326–35.
Geyer HL, Emanuel RM, Dueck AC, et al. Distinct clustering of symptomatic burden amongst myeloproliferative neoplasm patients: retrospective assessment in 1470 patients. Blood 2014. This article details the results of a large scale analysis of MPN-related symptoms and groups disease subtypes into specific clusters which manifest similar symptom burden.
Geyer HL, Scherber R, Dueck A, et al. Gender differences and MPN symptom burden: An analysis by the MPN quality of life international study group (MPN-QOL ISG). European Hematology Association Annual Meeting Abstracts 2014;Abstract 1039.
Geyer H, Dueck AC, Emanuel RM, et al. Insomnia, quality of life and MPN symptom burden: an analysis by the MPN Quality of Life International Study Group (MPN-QOL ISG). Blood. 2013;122:4087.
Geyer H, Dueck AC, Emanuel RM, et al. Sexuality challenges, intimacy, and MPN symptom burden: an analysis by the MPN Quality of Life International Study Group (MPN-QOL ISG). Blood. 2013;122:4088.
L. P, Dueck A, Bennett R, et al. Living With Cancer: Assessing The Educational, Symptomatic, and Quality Of Life Impact Of a Comprehensive Patient Symposia On Cancer Patients Blood 2013;122:a2955.
Passamonti F, Thiele J, Girodon F, et al. A prognostic model to predict survival in 867 World Health Organization-defined essential thrombocythemia at diagnosis: a study by the International Working Group on Myelofibrosis Research and Treatment. Blood. 2012;120:1197–201.
Compliance with Ethics Guidelines
Conflict of Interest
Dr. Robyn M. Scherber and Dr. Holly L. Geyer each declare no potential conflicts of interest.
Dr. Ruben A. Mesa is the Editor-in-Chief of Current Hematologic Malignancy Reports.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scherber, R.M., Geyer, H.L. & Mesa, R.A. Quality of Life in MPN Comes of Age as a Therapeutic Target. Curr Hematol Malig Rep 9, 324–330 (2014). https://doi.org/10.1007/s11899-014-0239-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-014-0239-9