Abstract
Purpose of Review
Non-ischaemic dilated cardiomyopathy (DCM) occurs in 1 in 2500 individuals in the general population and is associated with considerable morbidity and mortality. Studies involving large numbers of unselected DCM patients have led to consensus guidelines recommending implantable cardioverter-defibrillator (ICD) implantation for protection against sudden cardiac death (SCD) in those with LVEF ≤35%. The purpose of this article is to review the literature for other potential markers including serological, electrocardiographic, echocardiographic, cardiac magnetic resonance, ambulatory ECG and genetic data, to highlight other potential markers that may optimise risk stratification for SCD in this cohort and thereby allow a more personalized approach to ICD-implantation.
Recent Findings
Recent studies including the Danish study to assess the efficacy of ICDs in patients with non-ischemic systolic heart failure on mortality (DANISH) trial have questioned the benefits of ICD implantation in this group of patients with no changes in all-cause mortality. Recent pooled cohorts of patients with genetic DCM and in particular in those with Lamin A/C (LMNA) mutations have identified patients at increased risk of SCD and allowed the creation of algorithms to prognosticate SCD risk in mutation carriers. Furthermore, genetic testing has identified other DCM-causing genes including filamin C (FLNC) and RBM20 which may be associated with higher rates of ventricular arrhythmia.
Summary
To date, risk-stratification for SCD has been hampered by the utilisation of heterogenous subsets of idiopathic DCM patients and by use of static risk models where predictions are based on a single time point with a lack of consideration of disease progression. The current focus of personalised risk-stratification for SCD is shifting towards better characterisation of underlying DCM aetiology and the development of multi-parametric risk-stratification models that incorporate time-dependent disease characteristics and novel biomarkers.
Similar content being viewed by others
Introduction
Non-ischaemic dilated cardiomyopathy (DCM) occurs in 1 in 2500 individuals in the general population [1•] and predisposes to end-stage heart failure (ESHF) and malignant ventricular arrhythmia (VA). The annual incidence of sudden cardiac death (SCD) in DCM is 2-4% with sudden death accounting for up to half of all deaths [2, 3]. This is also reflected in registry data of survivors of an aborted cardiac arrest, where DCM is the underlying aetiology in 10-19% [4]. Importantly, SCD may be the initial manifestation of DCM in previously asymptomatic individuals.
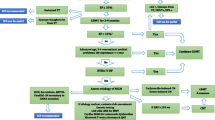
While most data suggest that implantable cardioverter defibrillators (ICD) prevent SCD and lower mortality in DCM cohorts by up to 31% [5], only 20-25% of patients with primary prevention ICD receive an appropriate shock within 5 years of device implantation highlighting that current risk stratification algorithms remain suboptimal [6, 7]. Most studies examining predictors of SCD in DCM populations have focussed on left ventricular ejection fraction (LVEF) as a stratifier. However, whilst it is true that SCD events are relatively numerous in those with severe LV systolic impairment, the proportion of SCDs compared to other causes of cardiovascular mortality such as ESHF is much greater in individuals with milder reductions in LVEF. The resultant paradox is that many patients presenting with SCD have a LVEF that does not meet consensus criteria for primary prevention ICD implantation and often in those without preceding symptoms of heart failure [8]. Moreover, a third of adverse events can occur late (>72 months) post symptom onset, despite optimal medical therapy (OMT) [9].
Given these limitations of LVEF as a solitary risk marker in DCM, alternative biochemical, clinical and imaging risk markers have been sought. As yet, most studies are based on retrospective or observational registry data, but a number of potential candidates for risk predictors are emerging and discussed below.
Serological markers
Troponin T (TnT) and NT-proBNP (BNP) are serum biomarkers that indicate the severity of the heart failure phenotype and/or myocardial injury and are correlated with prognosis [10, 11]. Raised BNP levels have been shown to predict SCD risk with a relative risk of 3.7 [12] and in patients with ICDs, raised BNP levels are associated with an over two-fold increase in the risk of VA [12, 13].
C-reactive protein (CRP) is a marker of inflammation, and significantly higher levels have been shown to be present in DCM patients dying within 5 years of hospitalization compared to survivors [14]. In those with acute heart failure necessitating hospitalization, CRP levels are also associated with an increased likelihood of intensive care admission and in-hospital mortality [15]. Elevated high-sensitivity CRP (hs-CRP) levels are associated with all-cause mortality and rehospitalization in DCM although not with SCD specifically [16].
Serum levels of pro-inflammatory cytokines, including IL-1, are significantly increased in patients with heart failure [17, 18]. Interleukin 1-B has been demonstrated to affect cardiac remodelling and contributes to post-inflammatory myocardial fibrosis and DCM in animal models [19]. Increasing IL-1 levels are associated with progressive severities of heart failure and atrial fibrillation [20]. ST2, another cytokine, has protective actions against myocyte fibrosis and soluble ST2 receptor is released in response to myocardial stress [18]. An increase in soluble ST2 levels has been demonstrated to be an independent predictor of one-year mortality in acute decompensated heart failure [21] and of hospitalizations and mortality in chronic heart failure. However, its usage in prognostic models did not demonstrate a significantly improved means of risk reclassification [22]. Currently, evidence to demonstrate the routine use of cytokine levels for SCD prognostication is lacking.
Electrocardiographic markers
Several studies have analysed the association of various electrocardiographic markers with the risk of SCD in DCM including QRS duration, QRS fragmentation, microvolt T-wave alternans and QRS-T angle. In a detailed meta-analysis of markers of SCD, both depolarization and repolarization ECG abnormalities were more common in DCM patients with SCD. Fragmented QRS complexes, T-wave alternans, abnormal signal-averaged ECG (SAECG) and prolonged QRS duration were all significantly associated with SCD with decreasing magnitudes of relative risk [23].
QRS duration is a relatively easy, reproducible marker and has been shown to be a significant predictor of cardiovascular mortality, SCD and appropriate ICD shock in patients with DCM [24]. In a study of 710 DCM patients, QRS duration at baseline evaluation was independently associated with arrhythmic endpoints (SCD, aborted SCD, appropriate ICD therapy or sustained VT) at one-year [9]. Similar conclusions were identified in sub-group analysis of the SCD-HeFT cohort where patients with QRS≥120ms had greater survival benefit with ICD compared to placebo therapy although this was not stratified according to ischaemic or non-ischaemic cardiomyopathy subtypes [7]. Other studies have utilised a combination of ECG and imaging predictors to optimise risk stratification. For example, in a study of 531 patients with idiopathic DCM, a combination of broad QRS (QRS duration ≥120ms) together with late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR) provided incremental benefit to LGE alone in predicting all-cause mortality (HR 4.3; 95%CI 1.2-15.5; p=0.03) [25]. QRS fragmentation is also more common in patients with chronic heart failure and SCD [26] and is associated with all-cause mortality and arrhythmic events, independent of QRS duration [26, 27].
Microvolt T-wave alternans (TWA) is defined as a beat-to-beat alternation of T wave amplitude and reflects spatiotemporal repolarization heterogeneity which acts as a substrate for arrhythmia. TWA was initially thought to be a promising independent marker of SCD in patients with DCM [28,29,30,31], but conflicting data including a negative prospective substudy analysis of SCD-HeFT trial involving 490 patient have dampened enthusiasm for its use as an important electrocardiographic marker [32, 33].
Ambulatory ECG Monitoring
24-hour Holter monitoring demonstrates non-sustained ventricular tachycardia (NSVT) in 40-60% of patients and polymorphic ventricular ectopy (VE) in up to 90% of DCM patients [34]. Data from electro-anatomical mapping catheter studies suggest that up to 80% of these ventricular arrhythmias are secondary to scar-related re-entry into the myocardium [35, 36]. However, in a study of 319 idiopathic DCM patients on OMT, NSVT was not found to be predictive of malignant VA (SCD, Sustained VT, ventricular fibrillation (VF), appropriate ICD shock) in patients with LVEF ≤35%. In those with LVEF >35%, however, the number of runs and length of NSVT were predictive of VA (HR 5.3, 95%CI: 1.6-17.9) [37]. Other studies of patients with moderate-severe heart failure failed to demonstrate that NSVT provided any incremental benefit in predicting patients at risk of SCD [38].
Sustained ventricular tachycardia (VT) occurs in under 5% of DCM patients and is a risk factor for SCD. Inducibility of VT on electrophysiology study (EPS) has been suggested as valuable for risk stratification in previous consensus guidelines, but most data suggest that EPS discriminate poorly between high and low-risk patients with respect to SCD. In the MADIT II study, inducibility of ventricular arrhythmia on EPS was associated with an increased likelihood of VT but not of VF and was not a good predictor of a composite endpoint of VT/VF [39, 40]. In current clinical practice, EPS is not routinely used to guide decision making on ICD implantation.
Echocardiographic Risk Markers
Echocardiography is the most common imaging modality used to assess the severity of LVEF and thereby provide prognostic data in patients with DCM. Other echocardiographic parameters have also been considered including LV dimension, atrial size and in a study of 710 DCM patients, mitral regurgitation on baseline TTE was an important predictor of VA [9].
Newer echocardiographic technologies such a speckle tracking, have been suggested to provide incremental improvements in SCD stratification. For example, global longitudinal strain (GLS) is a marker of myocardial regional contractility and an impaired GLS may reflect myocardial fibrosis [41]. GLS can also identify subtle motion abnormalities in the ventricle prior to more overt changes or remodelling resulting in impairment in LVEF. Studies have demonstrated that impaired GLS is associated with increased arrhythmic events (sustained VT and cardiac arrest) (HR 1.3; 95%CI 1.1-1.5; p=0.01) and may be more predictive for arrhythmic events than LVEF [42].
Mechanical dispersion is the standard deviation between different myocardial segments of the time to peak negative strain. In a small study of 94 patients with DCM, mechanical dispersion was higher in patients that experienced arrhythmic events compared to those that didn’t (98±43ms vs 56±18ms) and was an independent predictor of arrhythmias (HR 1.28; 95%CI 1.1-1.5) [42].
Cardiac Magnetic Resonance (CMR) Markers
CMR provides a reproducible assessment of LVEF and LV volumes, and with the administration of gadolinium contrast, data on myocardial scarring. T1 and T2 sequences are also used to quantify interstitial fibrosis and myocardial oedema.
Late gadolinium enhancement (LGE) is present in up to 38% of DCM patients [43]. Myocardial fibrosis can occur due to collagen accumulation resulting in interstitial expansion without myocardial necrosis (interstitial fibrosis) and from cardiomyocyte death (replacement fibrosis) [44]. Myocardial fibrosis is a substrate for VA in ischaemic heart disease where the scar represents a transition point between normal myocardium and fibrotic tissue resulting in the creation of slow-conduction re-entry circuits and ‘scar-related’ VT. This mechanism may also contribute to VT in some patients with DCM [45].
Several studies have demonstrated an association between fibrosis and SCD in patients with DCM [43, 46,47,48,49,50]. In the largest meta-analysis, incorporating 2948 DCM patients, LGE was significantly associated with an arrhythmic endpoint (OR 4.3, p<0.001) [51]. An arrhythmic endpoint occurred in 21% of patients with LGE (annual event rate of 6.9%) compared to 4.7% of patients (annual event rate of 1.6%) without LGE. Importantly, in the sub-category of patients with DCM and LVEF>35%, LGE was demonstrated to be significantly associated with an arrhythmic endpoint (OR 5.2, 95%CI: 3.4-7.9; p<0.001) [51].
Despite these extensive data, there are no randomised controlled trial (RCT) data to confirm the impact of these findings with respect to LGE and there is no conclusive evidence to demonstrate that patients with severe LVSD and without fibrosis are not at-risk of SCD. Other issues include a lack of standardization in the methodology, quantification and analysis of LGE as well as a lack of appreciable threshold of fibrosis over which arrhythmic risk elevates [52].
Some patients with DCM undergo endomyocardial biopsy (EMB) particularly if there are concerns of inflammatory conditions such as active myocarditis. EMB data may be utilised to provide data on the quantification of interstitial myocardial fibrosis in patients with DCM. This however is limited by procedural risks of EMB as well as by sampling error which may be particularly relevant with non-homogenous distributions of fibrosis. Native pre-contrast T1 times and extra-cellular volume fractions on cardiac magnetic resonance may reflect the degree of interstitial fibrosis in patients with DCM and offer a non-invasive method to quantify myocardial collagen composition [50, 53, 54]. In a multicentre prospective observational study involving 637 patients with DCM, T1-mapping indices were associated with all-cause mortality (p<0.001) [55]. Native T1 remained an independent predictor of all-cause mortality and of heart failure endpoints (hospitalization or ESHF-mortality) (HR 1.1; 95%CI: 1.1-1.2, p<0.001) [55]. However, this study did not analyse arrhythmic endpoints including SCD. In another single-centre study of 130 patients with ICDs and a mixture of ischaemic and non-ischaemic cardiomyopathy, native T1 value was an independent predictor of an arrhythmic endpoint consisting of appropriate ICD therapy or sustained VA (HR 1.1; 95%CI: 1.0-1.2) [56]. Further data is needed in unselected DCM cohorts to conclusively demonstrate the value of this modality in risk-stratification.
Autonomic Dysfunction
Patients with heart failure often have altered sympathetic activation which predisposes to progressive ventricular dysfunction and arrhythmias and is likely to contribute to an increased risk of SCD [57]. Myocardial uptake of 123-metaiodobenzylguanidine (MIBG), a nuclear tracer with the same uptake and storage as norepinephrine, is reduced in heart failure and cardiac MIBG washout rate has been associated with SCD in patients with mild or moderate heart failure with LVEF <40% [58,59,60,61]. However, in a meta-analysis of various predictors of SCD in DCM, autonomic variables such as heart rate variability, heart rate turbulence and baroreflex sensitivity were not demonstrated to be significantly associated with SCD [23].
Genetic Markers
A family history of sudden cardiac death is associated with SCD events in heart failure patients [62] and more than 1 in 4 patients with DCM carry a pathogenic genetic mutation. In a recent study of 487 DCM patients undergoing genetic testing, there was a trend towards increased malignant ventricular arrhythmia (SCD / VT / VF) in gene variant carriers (p=0.06) and increased heart-failure events (destination left ventricular assist device, heart transplantation or ESHF-mortality) [63]. This study demonstrated that LMNA or desmosomal mutation-carriers were at greatest risk of VA regardless of LVEF [63].
The number of disease-causing genes is considerable, but emerging data suggest that truncating mutations in titin (TTNtv) are the commonest cause of genetic DCM, accounting for between 15-25% of cases [64]. Patients with TTNtv appear to have high rates of positive remodelling on OMT and similar outcomes to patients without TTN mutations [65, 66]. Arrhythmic deaths do occur in TTN mutation carriers, but progressive heart failure predominates. In contrast, other disease genes appear to be associated with a much higher risk of SCD. Specific examples include the following:
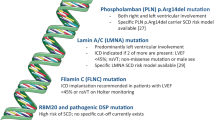
Lamin AC (LMNA)
LMNA mutations account for as many as 10% of cases of genetic DCM and are associated with early atrial and ventricular arrhythmia, premature conduction disease, SCD and progression to ESHF resulting in death or heart transplantation (HTx) [67,68,69]. LMNA mutations are associated with a high clinical penetrance by the age of 60 years and with considerable morbidity and mortality [70]. In a study of 269 LMNA mutation carriers followed up for a median of 43 months, 18% experienced malignant VA (4% an aborted SCD event, 9% an appropriate ICD therapy and over 4% had a SCD). This study identified the NSVT, male sex, non-missense mutations and LVEF <45% as risk factors for malignant VA [71]. A more contemporary study involving 839 LMNA mutation carriers identified the same risk factors but also found 1st degree or higher AV block as a predictor [72]. This study and others highlight that conventional EF-threshold based guidelines for ICD implantation are inappropriate in LMNA mutation carriers and that higher EF thresholds should be accepted for ICD implantation.
Filamin C (FLNC)
Truncating mutations in FLNC account for up to 4% of DCM cases [73] and are associated with frequent ventricular arrhythmia (82%). In one study, 15% of patients with FLNC mutations had a cardiac arrest with a mean age of 42±16 years and a mean LVEF of 39.6±12% [73]. Moreover, 20% of patients with a primary prevention ICD had an appropriate ICD shock, much higher than that seen in unselected DCM populations [73]. Although larger data sets with longitudinal follow-up are needed, preliminary data suggests that FLNC mutations are associated with a significant risk of VA events and lower thresholds for ICD implantation may need to be considered in this group.
RNA-binding motif protein 20 (RBM20)
The RBM20 splicing factor is involved in the splicing of Titin (TTN) and calcium/calmodulin dependent kinase II delta (CAMK2D). A recent study of carriers of RBM20 mutations identified an association with an arrhythmogenic DCM with increased incidence of VA [74]. 44% of carriers of RBM20 mutations had sustained VA compared to 5% of TTN mutation carriers despite similar LVEF. In another multicentre study of 74 RBM20 mutation carriers, there was a considerable family history of SCD (51%) and patients with RBM20 mutations were more likely to have NSVT (43% vs 11%) and sustained VT (25% vs 2%) than idiopathic DCM cohorts [75].
Phospholamban (PLN)
Mutations in the phospholamban gene are associated with an arrhythmogenic DCM characterised by low voltage ECG complexes and frequent ventricular arrhythmic events. The best characterised PLN variant is R14del which is a founder mutation in the Netherlands that accounts for 15% of DCM regionally. DCM patients with R14del are more likely to have an appropriate ICD shock (47% vs 10%) and heart transplantation (18% vs 2%) compared to those that do not carry a PLN mutation. R14del carriers are also more likely to have a family history of SCD at <50 years (36% vs 16%) with a mean age of SCD at 37.7 years; SCD often being the index presentation at a young age [76].
Desmoplakin (DSP)
Mutations in the DSP gene are associated with ARVC but recent data suggest that they more typically cause a left-dominant arrhythmogenic cardiomyopathy. Variants in DSP are associated with left ventricular systolic dysfunction, myocardial fibrosis and ventricular arrhythmia [77]. Importantly, carriers of DSP mutations can develop sustained VT in the absence of severe LVSD and primary prevention ICD implantation is often considered in the presence of an abnormal LVEF [77].
Sodium Voltage-Gated Channel Alpha Subunit 5 (SCN5A)
Mutations in SCN5A are associated with conduction disease and Brugada syndrome. Some mutations, including R222Q are associated with DCM, atrial arrhythmia, frequent premature ventricular complexes and SCD [78, 79]. Arrhythmias are reported in over 90% of SCN5A DCM cases [80]. There are reports of successful arrhythmia and ectopy treatment with quinidine in R222Q mutation carriers with improvement in LVEF [81].
Conclusions: Implications for Primary Prevention ICD Therapy in DCM
Risk stratification for SCD in DCM is difficult and complex. Whilst SCD can be prevented by implantation of primary prevention ICDs, these devices come with their own risks.
ICD implantation can be associated with short and long-term complications including device infection or erosion, psychological impact and a risk of inappropriate shock. This also includes the risk of device malfunction with lead failure and the need for regular generator changes over the patient’s lifetime [82,83,84]. Given these complications from ICDs and that patients with DCM are often younger than their ischaemic counterparts, there is a need for better risk-stratification in DCM to identify those at elevated risk of SCD.
All RCTs for the prevention of SCD in DCM have focussed on LVEF as a stratifier of risk [2, 7]. Consequently, consensus guidelines for ICD implantation in DCM are limited to patients in New York Heart Association (NYHA) class II-III heart failure and with LVEF <35% [85, 86, 87••]. With respect to individual studies, there is some difference in the reported impact of ICD on all-cause mortality. In the DEFINITE trial, ICD implantation significantly reduced the risk of arrhythmic SCD but not all-cause mortality [2]. In contrast, the SCD-HeFT trial demonstrated that ICD implantation resulted in a reduction in all-cause mortality in a mixed cohort of patients with ischaemic and non-ischaemic causes of heart failure [7]. In a much quoted meta-analysis of five RCTs incorporating 1854 patients with DCM, there was a significant reduction in all-cause mortality with ICD implantation compared to OMT [5].
Most recently, The DANISH trial demonstrated that ICD implantation in DCM (76% idiopathic) was not associated with a significant reduction in all-cause mortality compared to OMT (21.6% vs 23.4%, respectively) in spite of a reduction in SCD rates [88••]. Possible explanations for this include a low SCD event rate (<5%) in the DANISH trial as well as excellent OMT with >90% of patients on ACE inhibitors / Beta-blockers and >50% on mineralocorticoid antagonists or with cardiac resynchronization therapy (CRT) [88••]. As yet, these findings have not resulted in a change in current practice guidelines for the use of ICD in DCM, but they do illustrate the need for more individualised risk stratification in DCM cohorts.
Risk stratification has been hampered by utilisation of heterogenous subsets of idiopathic DCM patients and a by a static risk assessment where predictions are based on a single time point with a lack of consideration of progression (deterioration or improvement) in the condition on OMT. The current focus is shifting towards better characterisation of the underlying aetiology of DCM and the development of multi-parametric risk-stratification models that incorporate time-dependent disease characteristics and novel biomarkers.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• McNally EM, Mestroni L. Dilated Cardiomyopathy: Genetic Determinants and Mechanisms. Circ Res. 2017;121(7):731–48. This review focuses on different genetic mutations that are associated with increased risk of arrhythmia and may impact on prognosis.
Kadish A, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151–8.
Grimm W, Maisch B. Sudden cardiac death in dilated cardiomyopathy -- therapeutic options. Herz. 2002;27(8):750–9.
Deo R, Albert CM. Epidemiology and genetics of sudden cardiac death. Circulation. 2012;125(4):620–37.
Desai AS, et al. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: a meta-analysis of randomized controlled trials. JAMA. 2004;292(23):2874–9.
Levy WC, et al. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation. 2009;120(10):835–42.
Bardy GH, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–37.
Gorgels AP, et al. Out-of-hospital cardiac arrest--the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24(13):1204–9.
Stolfo D, et al. Arrhythmic Risk Stratification in Patients With Idiopathic Dilated Cardiomyopathy. Am J Cardiol. 2018;121(12):1601–9.
Vrtovec B, et al. Relation of B-type natriuretic peptide level in heart failure to sudden cardiac death in patients with and without QT interval prolongation. Am J Cardiol. 2013;111(6):886–90.
Nakamura H, et al. Cardiac troponin T as a predictor of cardiac death in patients with left ventricular dysfunction. J Arrhythm. 2017;33(5):463–8.
Scott PA, et al. Brain natriuretic peptide for the prediction of sudden cardiac death and ventricular arrhythmias: a meta-analysis. Eur J Heart Fail. 2009;11(10):958–66.
Levine YC, et al. B-type natriuretic peptide is a major predictor of ventricular tachyarrhythmias. Heart Rhythm. 2014;11(7):1109–16.
Kaneko K, et al. C-Reactive protein in dilated cardiomyopathy. Cardiology. 1999;91(4):215–9.
Mueller C, et al. Inflammation and long-term mortality in acute congestive heart failure. Am Heart J. 2006;151(4):845–50.
Sadahiro T, et al. MRI and serum high-sensitivity C reactive protein predict long-term mortality in non-ischaemic cardiomyopathy. Open Heart. 2015;2(1):e000298.
Yndestad A, et al. Systemic inflammation in heart failure--the whys and wherefores. Heart Fail Rev. 2006;11(1):83–92.
Szekely Y, Arbel Y. A Review of Interleukin-1 in Heart Disease: Where Do We Stand Today? Cardiol Ther. 2018;7(1):25–44.
Blyszczuk P, et al. Myeloid differentiation factor-88/interleukin-1 signaling controls cardiac fibrosis and heart failure progression in inflammatory dilated cardiomyopathy. Circ Res. 2009;105(9):912–20.
Cheng T, et al. Correlation between atrial fibrillation, serum amyloid protein A and other inflammatory cytokines. Mol Med Rep. 2012;6(3):581–4.
Anand IS, et al. Prognostic value of soluble ST2 in the Valsartan Heart Failure Trial. Circ Heart Fail. 2014;7(3):418–26.
Felker GM, et al. Soluble ST2 in ambulatory patients with heart failure: Association with functional capacity and long-term outcomes. Circ Heart Fail. 2013;6(6):1172–9.
Goldberger JJ, et al. Sudden cardiac death risk stratification in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2014;63(18):1879–89.
Hombach V, et al. Electrocardiographic and cardiac magnetic resonance imaging parameters as predictors of a worse outcome in patients with idiopathic dilated cardiomyopathy. Eur Heart J. 2009;30(16):2011–8.
Marume K, et al. Mortality and Sudden Cardiac Death Risk Stratification Using the Noninvasive Combination of Wide QRS Duration and Late Gadolinium Enhancement in Idiopathic Dilated Cardiomyopathy. Circ Arrhythm Electrophysiol. 2018;11(4):e006233.
Pei J, et al. The J wave and fragmented QRS complexes in inferior leads associated with sudden cardiac death in patients with chronic heart failure. Europace. 2012;14(8):1180–7.
Sha J, et al. Fragmented QRS is associated with all-cause mortality and ventricular arrhythmias in patient with idiopathic dilated cardiomyopathy. Ann Noninvasive Electrocardiol. 2011;16(3):270–5.
Kitamura H, et al. Onset heart rate of microvolt-level T-wave alternans provides clinical and prognostic value in nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2002;39(2):295–300.
Hohnloser SH, et al. Usefulness of microvolt T-wave alternans for prediction of ventricular tachyarrhythmic events in patients with dilated cardiomyopathy: results from a prospective observational study. J Am Coll Cardiol. 2003;41(12):2220–4.
Salerno-Uriarte JA, et al. Prognostic value of T-wave alternans in patients with heart failure due to nonischemic cardiomyopathy: results of the ALPHA Study. J Am Coll Cardiol. 2007;50(19):1896–904.
Klingenheben T, et al. Predictive value of T-wave alternans for arrhythmic events in patients with congestive heart failure. Lancet. 2000;356(9230):651–2.
Grimm W, et al. Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: results of the Marburg Cardiomyopathy Study. Circulation. 2003;108(23):2883–91.
Gold MR, et al. Role of microvolt T-wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: primary results from the T-wave alternans sudden cardiac death in heart failure trial substudy. Circulation. 2008;118(20):2022–8.
Liuba I, Marchlinski FE. The substrate and ablation of ventricular tachycardia in patients with nonischemic cardiomyopathy. Circ J. 2013;77(8):1957–66.
Stevenson WG, Soejima K. Catheter ablation for ventricular tachycardia. Circulation. 2007;115(21):2750–60.
Soejima K, et al. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: the importance of low-voltage scars. J Am Coll Cardiol. 2004;43(10):1834–42.
Zecchin M, et al. Are nonsustained ventricular tachycardias predictive of major arrhythmias in patients with dilated cardiomyopathy on optimal medical treatment? Pacing Clin Electrophysiol. 2008;31(3):290–9.
Teerlink JR, et al. Ambulatory ventricular arrhythmias in patients with heart failure do not specifically predict an increased risk of sudden death. PROMISE (Prospective Randomized Milrinone Survival Evaluation) Investigators. Circulation. 2000;101(1):40–6.
Daubert JP, et al. Predictive value of ventricular arrhythmia inducibility for subsequent ventricular tachycardia or ventricular fibrillation in Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J Am Coll Cardiol. 2006;47(1):98–107.
Chen X, et al. Role of programmed ventricular stimulation in patients with idiopathic dilated cardiomyopathy and documented sustained ventricular tachyarrhythmias: inducibility and prognostic value in 102 patients. Eur Heart J. 1994;15(1):76–82.
Masarone D, et al. Risk Stratification of Sudden Cardiac Death in Patients with Heart Failure: An update. J Clin Med. 2018;7(11):436.
Haugaa KH, et al. Risk assessment of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy by strain echocardiography. J Am Soc Echocardiogr. 2012;25(6):667–73.
Kuruvilla S, et al. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2014;7(2):250–8.
Beltrami CA, et al. The cellular basis of dilated cardiomyopathy in humans. J Mol Cell Cardiol. 1995;27(1):291–305.
Bogun FM, et al. Delayed-enhanced magnetic resonance imaging in nonischemic cardiomyopathy: utility for identifying the ventricular arrhythmia substrate. J Am Coll Cardiol. 2009;53(13):1138–45.
Masci PG, et al. Incremental prognostic value of myocardial fibrosis in patients with non-ischemic cardiomyopathy without congestive heart failure. Circ Heart Fail. 2014;7(3):448–56.
Gulati A, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309(9):896–908.
Disertori M, et al. Myocardial Fibrosis Assessment by LGE Is a Powerful Predictor of Ventricular Tachyarrhythmias in Ischemic and Nonischemic LV Dysfunction: A Meta-Analysis. JACC Cardiovasc Imaging. 2016;9(9):1046–55.
Halliday BP, et al. Association Between Midwall Late Gadolinium Enhancement and Sudden Cardiac Death in Patients With Dilated Cardiomyopathy and Mild and Moderate Left Ventricular Systolic Dysfunction. Circulation. 2017;135(22):2106–15.
Halliday BP, et al. Personalizing Risk Stratification for Sudden Death in Dilated Cardiomyopathy: The Past, Present, and Future. Circulation. 2017;136(2):215–31.
Di Marco A, et al. Late Gadolinium Enhancement and the Risk for Ventricular Arrhythmias or Sudden Death in Dilated Cardiomyopathy: Systematic Review and Meta-Analysis. JACC Heart Fail. 2017;5(1):28–38.
Arbustini E, Disertori M, Narula J. Primary Prevention of Sudden Arrhythmic Death in Dilated Cardiomyopathy: Current Guidelines and Risk Stratification. JACC Heart Fail. 2017;5(1):39–43.
Flett AS, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122(2):138–44.
aus dem Siepen F, et al. T1 mapping in dilated cardiomyopathy with cardiac magnetic resonance: quantification of diffuse myocardial fibrosis and comparison with endomyocardial biopsy. Eur Heart J Cardiovasc Imaging. 2015;16(2):210–6.
Puntmann VO, et al. T1-Mapping and Outcome in Nonischemic Cardiomyopathy: All-Cause Mortality and Heart Failure. JACC Cardiovasc Imaging. 2016;9(1):40–50.
Chen Z, et al. Myocardial tissue characterization by cardiac magnetic resonance imaging using T1 mapping predicts ventricular arrhythmia in ischemic and non-ischemic cardiomyopathy patients with implantable cardioverter-defibrillators. Heart Rhythm. 2015;12(4):792–801.
Triposkiadis F, et al. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54(19):1747–62.
Merlet P, et al. Prognostic value of MIBG imaging in idiopathic dilated cardiomyopathy. J Nucl Med. 1999;40(6):917–23.
Schofer J, et al. Iodine-123 meta-iodobenzylguanidine scintigraphy: a noninvasive method to demonstrate myocardial adrenergic nervous system disintegrity in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1988;12(5):1252–8.
Tamaki S, et al. Cardiac iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction: results from a comparative study with signal-averaged electrocardiogram, heart rate variability, and QT dispersion. J Am Coll Cardiol. 2009;53(5):426–35.
Sood N, et al. Resting perfusion MPI-SPECT combined with cardiac 123I-mIBG sympathetic innervation imaging improves prediction of arrhythmic events in non-ischemic cardiomyopathy patients: sub-study from the ADMIRE-HF trial. J Nucl Cardiol. 2013;20(5):813–20.
Jouven X, et al. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999;99(15):1978–83.
Gigli M, et al. Genetic Risk of Arrhythmic Phenotypes in Patients With Dilated Cardiomyopathy. J Am Coll Cardiol. 2019;74(11):1480–90.
Herman DS, et al. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366(7):619–28.
Roberts AM, et al. Integrated allelic, transcriptional, and phenomic dissection of the cardiac effects of titin truncations in health and disease. Sci Transl Med. 2015;7(270):270–6.
Jansweijer JA, et al. Truncating titin mutations are associated with a mild and treatable form of dilated cardiomyopathy. Eur J Heart Fail. 2017;19(4):512–21.
Anselme F, et al. Implantable cardioverter-defibrillators in lamin A/C mutation carriers with cardiac conduction disorders. Heart Rhythm. 2013;10(10):1492–8.
Kumar S, et al. Long-Term Arrhythmic and Nonarrhythmic Outcomes of Lamin A/C Mutation Carriers. Journal of the American College of Cardiology. 2016;68(21):2299–307.
Holmstrom M, et al. Late gadolinium enhanced cardiovascular magnetic resonance of lamin A/C gene mutation related dilated cardiomyopathy. J Cardiovasc Magn Reson. 2011;13:30.
Pasotti M, et al. Long-term outcome and risk stratification in dilated cardiolaminopathies. J Am Coll Cardiol. 2008;52(15):1250–60.
van Rijsingen IA, et al. Risk factors for malignant ventricular arrhythmias in lamin a/c mutation carriers a European cohort study. J Am Coll Cardiol. 2012;59(5):493–500.
Wahbi K, et al. Development and validation of a new risk prediction score for life-threatening ventricular Tachyarrhythmias in Laminopathies. Circulation. 2019;140(4):293–302.
Ortiz-Genga MF, et al. Truncating FLNC Mutations Are Associated With High-Risk Dilated and Arrhythmogenic Cardiomyopathies. J Am Coll Cardiol. 2016;68(22):2440–51.
van den Hoogenhof MMG, et al. RBM20 Mutations Induce an Arrhythmogenic Dilated Cardiomyopathy Related to Disturbed Calcium Handling. Circulation. 2018;138(13):1330–42.
Parikh VN, et al. Regional Variation in RBM20 Causes a Highly Penetrant Arrhythmogenic Cardiomyopathy. Circ Heart Fail. 2019;12(3):e005371.
van der Zwaag PA, et al. Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur J Heart Fail. 2012;14(11):1199–207.
Lopez-Ayala JM, et al. Desmoplakin truncations and arrhythmogenic left ventricular cardiomyopathy: characterizing a phenotype. Europace. 2014;16(12):1838–46.
Laurent G, et al. Multifocal ectopic Purkinje-related premature contractions: a new SCN5A-related cardiac channelopathy. J Am Coll Cardiol. 2012;60(2):144–56.
McNair WP, et al. SCN5A mutations associate with arrhythmic dilated cardiomyopathy and commonly localize to the voltage-sensing mechanism. J Am Coll Cardiol. 2011;57(21):2160–8.
Wilde AAM, Amin AS. Clinical Spectrum of SCN5A Mutations: Long QT Syndrome, Brugada Syndrome, and Cardiomyopathy. JACC Clin Electrophysiol. 2018;4(5):569–79.
Zakrzewska-Koperska J, et al. Rapid and effective response of the R222Q SCN5A to quinidine treatment in a patient with Purkinje-related ventricular arrhythmia and familial dilated cardiomyopathy: a case report. BMC Med Genet. 2018;19(1):94.
Ezzat VA, et al. A systematic review of ICD complications in randomised controlled trials versus registries: is our 'real-world' data an underestimation? Open Heart. 2015;2(1):e000198.
Basu-Ray I, et al. Subcutaneous Versus Transvenous Implantable Defibrillator Therapy: A Meta-Analysis of Case-Control Studies. JACC Clin Electrophysiol. 2017;3(13):1475–83.
Sears SF, et al. Defibrillator shocks and their effect on objective and subjective patient outcomes: Results of the PainFree SST clinical trial. Heart Rhythm. 2018;15(5):734–40.
Ponikowski P, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail, 2016. 2016;18(8):891–975.
Yancy CW, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239.
•• Priori SG, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace. 2015;17(11):1601–87. This consensus statement provides recommendations on the management of patients with DCM with respect to sudden cardiac death.
•• Kober L, et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N Engl J Med. 2016;375(13):1221–30. This was an important randomised controlled trial on ICD implantation in patients with DCM and severely impaired LV systolic function compared to optimal medical therapy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
M. Akhtar and P.M. Elliott declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Myocardial Disease
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Akhtar, M., Elliott, P.M. Risk Stratification for Sudden Cardiac Death in Non-Ischaemic Dilated Cardiomyopathy. Curr Cardiol Rep 21, 155 (2019). https://doi.org/10.1007/s11886-019-1236-3
Published:
DOI: https://doi.org/10.1007/s11886-019-1236-3