Abstract
Purpose
This systematic narrative review describes and compares the development and operational approaches of monitoring systems without a clinical care component that collect patient-reported outcome (PRO) data from cancer survivors.
Methods
Searches were conducted using Medline, PubMed, PsycINFO, the Cochrane Library, CINAHL, Scopus, Joanna Briggs Institute EBP Database and Google Scholar (Advanced). Sources of grey literature and websites of relevant organisations were also searched for relevant published and unpublished material. Articles were included if they described the development (including piloting) of monitoring systems with ongoing recruitment that collect PRO at more than one time point, from 6 months post-diagnosis onward.
Results
The initial searches returned 7290 unique citations. After screening titles and abstracts, 39 full-text articles were retrieved for more detailed examination. Eleven articles were included in the review, representing seven international monitoring systems. Systems varied in their scope, implementation process, governance and administration, recruitment and data collection, consent rates, PRO collection, use of PRO and translation strategies.
Conclusions
The most suitable approach for setting-up and implementing a monitoring system for ongoing surveillance will differ depending on the unique requirements, aims and level of resourcing available within a particular context. Better specification and consideration of how PRO data will be used, for what purpose, and by whom, is required to inform effective translational strategies to improve outcomes for cancer survivors.
Implications for Cancer Survivors
The findings from this review may inform the future development of survivorship monitoring systems in varied environments, which in turn may improve practices that lead to better outcomes for survivors.
Similar content being viewed by others
References
American Cancer Society. Global Cancer Facts and Figures: 3rd Edn. Atlanta: American Cancer Society 2015.
Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomark Prev. 2016;25(1):16–27.
Ferlay J, Soerjomataram I, Dikshit R, Eser C, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2014; doi:10.1002/ijc.29210.
Aziz NM, Rowland JH. Trends and advances in cancer survivorship research: challenge and opportunity. Semin Radiat Oncol. 2003;13(3):248–66.
Grunfeld E, Earle CC, Stovall E. A framework for cancer survivorship research and translation to policy. Cancer Epidemiol Biomark Prev. 2011;20(10):2099–104.
de Moor JS, Mariotto AB, Parry C, Alfano CM, Padgett L, Kent EE, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomark Prev. 2013;22(4):561–70.
Aziz NM. Cancer survivorship research: challenge and opportunity. J Nutr. 2002;132(11 Suppl):3494S–503S.
Food US, Administration D. Guidance for industry patient-reported outcome measures: use in medical product development to support labeling claims. Fed Regist. 2009;74(35):65132–3.
Ganz PA, Land SR, Antonio C, Zheng P, Yothers G, Petersen L, et al. Cancer survivorship research: the challenge of recruiting adult long term cancer survivors from a cooperative clinical trials group. J Cancer Surviv. 2009;3(3):137–47. doi:10.1007/s11764-009-0093-2.
Glaser AW, Fraser LK, Corner J, Feltbower R, Morris EJ, Hartwell G, et al. Patient-reported outcomes of cancer survivors in England 1–5 years after diagnosis: a cross-sectional survey. BMJ Open. 2013;3(4):e002317.
Jensen RE, Snyder CF, Abernethy AP, Basch E, Potosky A, Roberts AC, et al. Review of electronic patient-reported outcome systems used in cancer clinical care. Proc Am Soc Clin Oncol. 2013;10(4):e215–22.
Thong MS, Mols F, Stein KD, Smith T, Coebergh JW, van de Poll-Franse LV. Population-based cancer registries for quality-of-life research: a work-in-progress resource for survivorship studies? Cancer. 2013;119(Suppl 11):2109–23. doi:10.1002/cncr.28056.
Arora NK, Hamilton AS, Potosky AL, Rowland JH, Aziz NM, Bellizzi KM, et al. Population-based survivorship research using cancer registries: a study of non-Hodgkin’s lymphoma survivors. J Cancer Surviv. 2007;1(1):49–63. doi:10.1007/s11764-007-0004-3.
Ganz PA, Earle CC, Goodwin PJ. Journal of clinical oncology update on progress in cancer survivorship care and research. J Clin Oncol. 2012;30(30):3655–6.
van de Poll-Franse LV, Horevoorts N, Eenbergen Mv, Denollet J, Roukema JA, Aaronson NK et al. Eenbergen Mv, Denollet J, Roukema JA, Aaronson NK et al. The patient reported outcomes following initial treatment and long term evaluation of survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer 2011;47(14):2188–2194. doi:10.1016/j.ejca.2011.04.034.
Lubeck DP, Litwin MS, Henning JM, Carroll PR. Measurement of health-related quality of life in men with prostate cancer: the CaPSURE database. Qual Life Res. 1997;6(5):385–92.
Lubeck DP, Litwin MS, Henning JM, Stier DM, Mazonson P, Fisk R, et al. The CaPSURE database: a methodology for clinical practice and research in prostate cancer. Urology. 1996;48(5):773–7. doi:10.1016/S0090-4295(96)00226-9.
Porten SP, Cooperberg MR, Konety BR, Carroll PR. The example of CaPSURE: lessons learned from a national disease registry. World J Urol. 2011;29(3):265–71. doi:10.1007/s00345-011-0658-3.
Daly BJ, Douglas SL, Foley H, Lipson A, Liou CF, Bowman K, et al. Psychosocial registry for persons with cancer: a method of facilitating quality of life and symptom research. Psychooncology. 2007;16(4):358–64.
Freeman D, Dickerson G, Perman M. Multi-institutional registry for prostate cancer radiosurgery: a prospective observational clinical trial. Front. 2014;4:369. doi:10.3389/fonc.2014.00369.
Ashley L, Jones H, Forman D, Newsham A, Brown J, Downing A, et al. Feasibility test of a UK-scalable electronic system for regular collection of patient-reported outcome measures and linkage with clinical cancer registry data: the electronic Patient-reported Outcomes from Cancer Survivors (ePOCS) system. BMC Med Inf Decis Mak. 2011;11:66. doi:10.1186/1472-6947-11-66.
Ashley L, Jones H, Thomas J, Forman D, Newsham A, Morris E, et al. Integrating cancer survivors' experiences into UK cancer registries: design and development of the ePOCS system (electronic Patient-reported Outcomes from Cancer Survivors). Br J Cancer. 2011;105(Suppl 1):S74–81. doi:10.1038/bjc.2011.424.
Ashley L, Jones H, Thomas J, Newsham A, Downing A, Morris E, et al. Integrating patient reported outcomes with clinical cancer registry data: a feasibility study of the electronic Patient-Reported Outcomes From Cancer Survivors (ePOCS) system. J Med Internet Res. 2013;15(10):e230. doi:10.2196/jmir.2764.
Evans SM, Millar JL, Wood JM, Davis ID, Bolton D, Giles GG, et al. The Prostate Cancer Registry: Monitoring patterns and quality of care for men diagnosed with prostate cancer. BJU Int. 2013;111(4 B):E158–E66. doi:10.1111/j.1464-410X.2012.11530.x.
Stirling RG, Evans S, McLaughlin P, Senthuren M, Millar J, Gooi J, et al. The Victorian Lung Cancer Registry pilot: improving the quality of lung cancer care through the use of a disease quality registry. Lung. 2014;192(5):749–58.
Howell D, Fitch M, Bakker D, Green E, Sussman J, Mayo S, et al. Core domains for a person-focused outcome measurement system in cancer (PROMS-Cancer Core) for routine care: a scoping review and Canadian Delphi Consensus. Value Health. 2013;16(1):76–87.
Chaudhry S, Jin L, Meltzer D. Use of a self-report-generated Charlson Comorbidity Index for predicting mortality. Med Care. 2005;43(6):607–15.
Litwin MS, Greenfield S, Elkin EP, Lubeck DP, Broering JM, Kaplan SH. Assessment of prognosis with the total illness burden index for prostate cancer: aiding clinicians in treatment choice. Cancer. 2007;109(9):1777–83.
Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Care Res (Hoboken). 2003;49(2):156–63.
Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky performance status scale: an examination of its reliability and validity in a research setting. Cancer. 1984;53(9):2002–7.
Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH. The use of the nitrogen mustards in the palliative treatment of carcinoma. With particular reference to bronchogenic carcinoma. Cancer. 1948;1(4):634–56.
Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32(7):1135–41.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–56.
Michielsen HJ, De Vries J, Van Heck GL, Van de Vijver FJ, Sijtsma K. Examination of the dimensionality of fatigue: the construction of the fatigue assessment scale (FAS). Eur J Psychol Assess. 2004;20(1):39.
Szymanski KM, Wei JT, Dunn RL, Sanda MG. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urology. 2010;76(5):1245–50.
Barry MJ, Fowler Jr FJ, O'Leary MP, Bruskewitz R, Holtgrewe H, Mebust W, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549–57. discussion 64
Rosen R, Cappelleri J, Smith M, Lipsky J, Pena B. Development and evaluation of an abridged, 5-item version of the International Index Of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11(6):319–26.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76.
van Andel G, Bottomley A, Fosså SD, Efficace F, Coens C, Guerif S, et al. An international field study of the EORTC QLQ-PR25: a questionnaire for assessing the health-related quality of life of patients with prostate cancer. Eur J Cancer. 2008;44(16):2418–24.
Gujral S, Conroy T, Fleissner C, Sezer O, King P, Avery K, et al. Assessing quality of life in patients with colorectal cancer: an update of the EORTC quality of life questionnaire. Eur J Cancer. 2007;43(10):1564–73.
Sprangers M, Groenvold M, Arraras JI, Franklin J, te Velde A, Muller M, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14(10):2756–68.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70.
Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D. Am J Prev Med. 1994.
Cella DF, Jacobsen PB, Orav EJ, Holland JC, Silberfarb PM, Rafla S. A brief POMS measure of distress for cancer patients. J Chronic Dis. 1987;40(10):939–42.
Moss-Morris R, Weinman J, Petrie K, Horne R, Cameron L, Buick D. The revised illness perception questionnaire (IPQ-R). Psychol Health. 2002;17(1):1–16.
Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy—spiritual well-being scale (FACIT-Sp). Ann Behav Med. 2002;24(1):49–58.
Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the life orientation test. J Pers Soc Psychol. 1994;67(6):1063.
Wright E, Kiely M, Johnston C, Smith A, Cull A, Selby P. Development and evaluation of an instrument to assess social difficulties in routine oncology practice. Qual Life Res. 2005;14(2):373–86.
Wright P, Smith AB, Keding A, Velikova G. The social difficulties inventory (SDI): development of subscales and scoring guidance for staff. Psychooncology. 2011;20(1):36–43. doi:10.1002/pon.1705.
Arraras JI, Kuljanic-Vlasic K, Bjordal K, Yun YH, Efficace F, Holzner B, et al. EORTC QLQ-INFO26: a questionnaire to assess information given to cancer patients a preliminary analysis in eight countries. Psycho-Oncology. 2007;16(3):249–54.
Funch DP, Marshall JR, Gebhardt GP. Assessment of a short scale to measure social support. Soc Sci Med. 1986;23(3):337–44.
Lubeck DP, Spitz PW, Fries JF, Wolfe F, Mitchell DM, Roth SH. A multicenter study of annual health service utilization and costs in rheumatoid arthritis. Arthritis Rheum. 1986;29(4):488–93.
Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: a review of its history, issues, progress, and documentation. J Rheumatol. 2003;30(1):167–78.
Bruce B, Fries JF. The Stanford health assessment questionnaire: dimensions and practical applications. Health Qual Life Outcomes. 2003;1(1):20.
Bruce B, Fries J. The health assessment questionnaire (HAQ). Clin Exp Rheumatol. 2005;23(5):S14.
EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health policy (Amsterdam, Netherlands). 1990;16(3):199.
Brooks R. EuroQol: the current state of play. Health policy. 1996;37(1):53–72.
Ware Jr JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992:473–83.
Ware J, Kosinski M, Dewey J. How to score version 2 of the SF-36 health survey. QualityMetric Incorporated: Lincoln, RI; 2000.
Ware Jr JE, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33.
Ware J, Kosinski M, Turner-Bowker D, Gandek B. SF-12v2: how to score version 2 of the SF-12 health survey. Lincoln, RI: QualityMetric Incorporated; 2002.
Ferrans CE. Definitions and conceptual models of quality of life. In: Lipscomb J, Gotay C, Snyder C, editors. Outcomes assessment in cancer. Cambridge: Cambridge University Press; 2005. p. 14–31.
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–9.
Avis NE, Smith KW, McGraw S, Smith RG, Petronis VM, Carver CS. Assessing quality of life in adult cancer survivors (QLACS). Qual Life Res. 2005;14(4):1007–23.
Nicolaije KA, Ezendam NP, Vos MC, Pijnenborg JM, Boll D, Boss EA, et al. Impact of an automatically generated cancer survivorship care plan on patient-reported outcomes in routine clinical practice: longitudinal outcomes of a pragmatic, cluster randomized trial. J Clin Oncol. 2015;33(31):3550–9.
Nicolaije KA, Husson O, Ezendam NP, Vos MC, Kruitwagen RF, Lybeert ML, et al. Endometrial cancer survivors are unsatisfied with received information about diagnosis, treatment and follow-up: a study from the population-based PROFILES registry. Patient Educ Couns. 2012;88(3):427–35. doi:10.1016/j.pec.2012.05.002.
Oerlemans S, Issa DE, van den Broek EC, Nijziel MR, Coebergh JWW, Huijgens PC, et al. Health-related quality of life and persistent symptoms in relation to (R-) CHOP14,(R-) CHOP21, and other therapies among patients with diffuse large B-cell lymphoma: results of the population-based PHAROS-registry. Ann Hematol. 2014;93(10):1705–15.
van de Poll-Franse LV, Pijnenborg JM, Boll D, Vos MC, van den Berg H, Lybeert ML, et al. Health related quality of life and symptoms after pelvic lymphadenectomy or radiotherapy vs. no adjuvant regional treatment in early-stage endometrial carcinoma: a large population-based study. Gynecol Oncol. 2012;127(1):153–60.
Sygna K, Johansen S, Ruland CM. Recruitment challenges in clinical research including cancer patients and their caregivers. A randomized controlled trial study and lessons learned. Trials. 2015;16:428. doi:10.1186/s13063-015-0948-y.
Treweek S, Lockhart P, Pitkethly M, Cook JA, Kjeldstrøm M, Johansen M et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3(2). doi: 10.1136/bmjopen-2012-002360.
Hunt KJ, Shlomo N, Addington-Hall J. Participant recruitment in sensitive surveys: a comparative trial of 'opt in' versus 'opt out' approaches. BMC Med Res Methodol. 2013;13:3. doi:10.1186/1471-2288-13-3.
Vellinga A, Cormican M, Hanahoe B, Bennett K, Murphy AW. Opt-out as an acceptable method of obtaining consent in medical research: a short report. BMC Med Res Methodol. 2011;11:40. doi:10.1186/1471-2288-11-40.
Olver IN. Linking data to improve health outcomes. Med J Aust. 2014;200(7):368–9.
Roder DM, Fong KM, Brown MP, Zalcberg J, Wainwright CE. Realising opportunities for evidence-based cancer service delivery and research: linking cancer registry and administrative data in Australia. Eur J Cancer Care (Engl). 2014;23(6):721–7. doi:10.1111/ecc.12242.
Roder D, Creighton N, Baker D, Walton R, Aranda S, Currow D. Changing roles of population-based cancer registries in Australia. Aust Health Rev. 2015;39(4):425–8. doi:10.1071/ah14250.
Ayanian JZ, Jacobsen PB. Enhancing research on cancer survivors. J Clin Oncol. 2006;24(32):5149–53.
Ganz PA. Why and how to study the fate of cancer survivors: observations from the clinic and the research laboratory. Eur J Cancer. 2003;39(15):2136–41.
Beskow LM, Sandler RS, Weinberger M. Research recruitment through US central cancer registries: balancing privacy and scientific issues. Am J Public Health. 2006;96(11):1920.
Barrett G, Cassell JA, Peacock JL, Coleman MP. National survey of British public's views on use of identifiable medical data by the National Cancer Registry. BMJ. 2006;332(7549):1068–72.
Smith TG, Castro KM, Troeschel AN, Arora NK, Lipscomb J, Jones SM, et al. The rationale for patient-reported outcomes surveillance in cancer and a reproducible method for achieving it. Cancer. 2016;122(3):344–51. doi:10.1002/cncr.29767.
Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013;309(8):814–22. doi:10.1001/jama.2013.879.
Calvert M, Blazeby J, Revicki D, Moher D, Brundage M. Reporting quality of life in clinical trials: a CONSORT extension. Lancet. 2011;378(9804):1684–5. doi:10.1016/s0140-6736(11)61256-7.
Brundage M, Blazeby J, Revicki D, Bass B, de Vet H, Duffy H, et al. Patient-reported outcomes in randomized clinical trials: development of ISOQOL reporting standards. Qual Life Res. 2013;22(6):1161–75. doi:10.1007/s11136-012-0252-1.
Basch E, Abernethy AP, Mullins CD, Reeve BB, Smith ML, Coons SJ, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30(34):4249–55. doi:10.1200/jco.2012.42.5967.
Reeve BB, Wyrwich KW, Wu AW, Velikova G, Terwee CB, Snyder CF, et al. ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Qual Life Res. 2013;22(8):1889–905. doi:10.1007/s11136-012-0344-y.
Journal of clinical epidemiology. 2010/08/06 ed 2010. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008; p. 1179–94.
Cella D, Stone AA. Health-related quality of life measurement in oncology: advances and opportunities. Am Psychol. 2015;70(2):175–85. doi:10.1037/a0037821.
European Organisation for Research and Treatment of Cancer. Assessing QoL of cancer survivors. http://www.eortc.org/research-groups/quality-of-life-group/strategy/. Accessed 2 August 2016.
Pereira J, Green E, Molloy S, Dudgeon D, Howell D, Krzyzanowska MK et al. Population-based standardized symptom screening: cancer care ontario's edmonton symptom assessment system and performance status initiatives. Journal of Oncology Practice. 2014;10(3).
Acknowledgements
We gratefully acknowledge the preparatory work carried out by Ms. Amber Halliday that informed the search strategy and methodology for this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding received.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
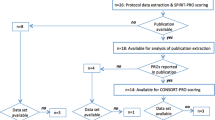
Figure S1
PRISMA flow diagram of study selection (DOCX 26.1 kb)
Table S1
Medline search executed June 15 2015 (DOCX 18 kb)
Table S2
Sources of grey literature (DOCX 19 kb)
Table S3
Monitoring system approaches and implementation (DOCX 39 kb)
Table S4
Validated patient-reported outcome measures used by the monitoring systems (DOCX 24.3 kb)
Rights and permissions
About this article
Cite this article
Corsini, N., Fish, J., Ramsey, I. et al. Cancer survivorship monitoring systems for the collection of patient-reported outcomes: a systematic narrative review of international approaches. J Cancer Surviv 11, 486–497 (2017). https://doi.org/10.1007/s11764-017-0607-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-017-0607-2