Abstract
Background
Laparoscopic gastric bypass (LGB) surgery markedly increases percent excess weight loss (%EWL) and obesity-related co-morbidities. However, poor study quality and minimal exploration of clinical, behavioral, and psychosocial mechanisms of weight loss have characterized research to date.
Methods
We conducted a comprehensive assessment of n=100 LGB patients surveyed 2–3 years following surgery using standardized measures.
Results
Mean %EWL at follow-up was 59.1±17.2%. This high level of weight loss was associated with a low rate of metabolic syndrome (10.6%), although medications were commonly used to achieve control. Mean adherence to daily vitamin and mineral supplements important to the management of LGB was only 57.6%, and suboptimal blood chemistry levels were found for ferritin (32% of patients), hematocrit (27%), thiamine (25%), and vitamin D (19%). Aerobic exercise level (R 2=0.08) and pre-surgical weight (R 2=0.04) were significantly associated with %EWL, but recommended eating style, fluid intake, clinic follow-up, and support group attendance were not. Psychosocial adjustment results showed an absence of symptomatic depression (0%), common use of antidepressant medications (32.0%), low emotional distress related to the post-surgical lifestyle (19.8±14.0; scale range 0–100), a high level of perceived benefit from weight loss in terms of functioning and emotional well-being (82.7±17.9; scale range 0–100), and a change in marital status for 26% of patients.
Conclusions
At 2–3 years following LGB surgery aerobic exercise, but not diet, fluid intake, or attendance at clinic visits or support groups, is associated with %EWL. Depression is symptomatically controlled by medications, lifestyle related distress is low, and marital status is significantly impacted.
Similar content being viewed by others
Introduction
Morbid obesity (MO) is associated with serious co-morbidities such as type 2 diabetes, metabolic syndrome, and sleep apnea [1]. Between 2000 and 2005, MO increased sharply by 52% and now affects 15 million adults [2]. Bariatric surgery has emerged as an important treatment option to reduce the co-morbidities associated with MO with approximately 220,000 operations carried out in 2008 [3].
Laparoscopic gastric bypass (LGB) has emerged as the preferred surgical procedure for MO in the USA based on its reported efficacy and complications profile [3]. Current estimates indicate that a mean percentage excess weight loss (%EWL) of 61.6–62.2% is achieved [4, 5]. However, recent reviews have identified significant concerns regarding LGB outcomes research reported to date, including variable study quality, lack of adequate long-term patient follow-up, high percentage of patients lost to follow-up, lack of standards for comparison of outcomes across studies, and poor statistical reporting [5, 6]. From a clinical perspective, gastric bypass surgery is traditionally not considered a “magic bullet” for weight loss but an adjunctive tool that helps the patient initiate and sustain new lifestyle behaviors that will support weight loss [7, 8]. For example, bariatric surgery procedures are not expected to override unhealthy eating habits that might re-emerge following surgery such as binge eating, grazing, emotional eating, night eating, drinking of high calorie beverages, or return to a sedentary lifestyle [9–12]. Moreover, patient adherence to the recommended regimen of clinical follow-up and support group attendance is considered important to weight loss success [9]. While there has been considerable clinical focus on multidisciplinary pre-surgical screening regarding medical, behavioral, and psychosocial suitability of candidates for bariatric surgery [14], there is a paucity of research examining post-surgical behavioral adaptation and clinical follow-up after surgery and their role in weight loss, particularly at 2 years or greater post-surgery when weight loss is thought to plateau [15]. Therefore, in this study, we examined the role of factors that have emerged from the clinical literature as potential mediators of weight loss outcome at 2–3 years following LGB surgery. These factors included post-surgical behavioral adherence, lifestyle-related emotional distress and depression, perceived benefits of weight loss, and the frequency of attendance to scheduled clinical follow-up visits and support groups provided by the clinical service.
Methods
Consecutive patients who received LGB or laparoscopic revision of prior LGB during the period April 2005 to January 2006 at the Baystate Medical Center Weight Loss Surgery (WLS) program participated in an initial cross-sectional study examining the prevalence of peri-operative symptoms [16]. This patient cohort was then approached 2 years post-surgery and invited to participate in the current study examining factors associated with weight loss at that time point. All patients were managed at the Baystate Medical Center (BMC) Weight Loss Surgery Clinic (WLSC), an American College of Surgeons Level 1A bariatric surgery Center of Excellence. BMC is a large, 641-bed teaching hospital in Western Massachusetts and the Western Campus of Tufts University School of Medicine. One hundred of 101 eligible patients (99.5%) agreed to participate in the original peri-operative study. These patients were contacted by the WLS staff again at a minimum of 2 years post-surgery, completed a new informed consent form approved by the BMC Institutional Review Board, and received a stipend for participation in the follow-up study.
Description of LGB Surgery
All patients underwent laparoscopic (Roux-en-Y) gastric bypass carried out by two WLSC surgeons (JR and JK), both of whom had fellowship training in minimally invasive surgery and had operated on over 250 cases at the time of the study. The same surgical technique was used for all patients, as follows: a 40-cm biliopancreatic limb was created, and a jejunojejunostomy was created with a linear stapler. The Roux limb was about 120 cm in each patient and was not varied based on BMI. A vertical gastric pouch was fashioned by starting perigastrically between the incisura and the left gastric artery, and then proceeding to the angle of His. The gastrojejunostomy was created by using a linear stapler, and the common enterotomy was stapled or sutured closed. The mesenteric defect of the jejunojejunostomy was closed in all patients; the Peterson’s defect was left open in this cohort of patients.
Demographic and Clinical Information
Patient data were obtained at baseline at the WLS clinic using a combination of questionnaires, laboratory blood tests, clinical exams, and chart review. This information included age, gender, race/ethnicity, educational level, marital status, work status, smoking status, patient’s goal weight, and height. Current weight and body fat percentage was assessed using a Tanita TBF-300A bio-electrical impedance analysis scale with maximum capacity of 600 lb and a 0% to 70% body fat range. Follow-up study assessments were carried out during the period January 2008 to August 2008 at which point the patients were assessed for marital status, weight and body composition, cardiovascular-related co-morbidities (diabetes, hypertension, and dyslipidemia), prescribed medications, post-surgical self-management behaviors (eating style, fluid intake, fruit and vegetable intake, protein intake, dumping syndrome management, supplement intake, and physical activity), emotional adjustment (post-surgical lifestyle, distress, and depression), perceived weight loss benefits, adherence to clinic follow-up visits, and support group attendance. Weight loss from baseline to follow-up was measured as %EWL defined as: ((weight loss/excess weight) × 100) and as percentage change in BMI in kg/m2 (%BML) expressed as ((BMI change/baseline BMI) × 100) [17]. “Successful surgery” was classified as %EWL > 50% based on the criteria of Oria [6]. Laboratory blood and urine tests focused on assessment of MO-related cardiovascular risk factors: diabetes (HbA1c ≥ 6%), hypercholesterolemia (LDL > 100 mg/dL and HDL < 40 mg/dL (men) or HDL < 50 mg/dL (women)), hypertension (blood pressure ≥140/90 mm/Hg), and metabolic syndrome based on Adult Treatment Panel III guidelines (waist circumference ≥102 cm (men) or ≥88 cm (women); triglycerides ≥ 150 mg/dL; HDL < 40 mg/dL (men) or <50 mg/dL (women); blood pressure ≥ 130/85 mm Hg; fasting blood glucose > 110 mg/dL) [18]. We also assessed renal function (hematocrit and BUN) and nutritional status (B12, thiamine, ferritin, iron, calcium, vitamin D, albumin for protein deficiency, and folic acid). Standard laboratory reference ranges used at the BMC Reference Laboratory and criteria obtained from the Longitudinal Assessment of Bariatric Surgery [19] were used to classify clinical and laboratory assessments. Smoking was assessed by two questions on current reported smoking status (yes/no) and number of cigarettes per day if a current smoker.
Behavioral and Psychosocial Measures
Patients were assessed 2 years post-surgery for behavioral and psychosocial factors that may be related to weight loss. Specifically, we assessed post-surgical self-management behaviors, depression, patient-perceived emotional distress related to the post-surgical treatment regimen, and perceptions of benefit from weight loss using published questionnaires shown to have satisfactory reliability and construct validity. Flesch–Kincaid reading level was below eighth grade (range 5.3 to 7.7) [20].
-
1.
Post-bariatric surgery self-management behaviors were assessed by the Bariatric Surgery Self-management Questionnaire [21]. The BSSQ assesses patient self-reported management behaviors carried out over the previous week. High adherence to these behaviors was expected by experienced clinicians to enhance patient success after surgery in terms of excess weight loss and adequate nutritional status (see Appendix). The seven BSSQ behavioral domains are (1) eating behaviors (eight EB items); (2) fluid intake (eight FI items); (3) protein intake (three PI items); (4) physical activity (three PA items); (5) dumping syndrome management (four DSM items); (6) fruit, vegetable, and whole grains intake (three FVW items); and (7) supplement intake (four SI items). BSSQ items have a Likert scale format of “never,” “sometimes,” “mostly,” or “always,” and subscale and total scores are converted to a 0–100 range for ease of interpretation, with higher scores indicating higher adherence. Internal reliability coefficients (coefficient alpha, α) for the BSSQ subscales are EB (α = 0.83), FI (α = 0.81), PI (α = 0.74), PA (α = 0.70), DSM (α = 0.79), FVW (α = 0.63), SI (α = 0.79), and total score (α = 0.83) [23]. Two-week test–retest reliabilities (intraclass coefficient (ICC)) for BSSQ subscales are EB (ICC = 0.72), FI (ICC = 0.68), PI (ICC = 0.60), PA (ICC = 0.54), DSM (ICC = 0.66), FVW (ICC = 0.46), SI (ICC = 0.66), and total score (ICC = 0.71) [21].
-
2.
Physical activity interview. In addition to completing the BSSQ PA subscale, patients were interviewed regarding their average weekly exercise as a component of total physical activity using a modified interview from the Nurses’ Health Study II [22]. Activities assessed in the NHS-II include walking, jogging, running, bicycling, aerobics/dance/rowing machine, tennis/racket sports, and swimming, with response categories available from 0 to >11 hours per week. Metabolic equivalent (MET) scores were assigned using the system described by Ainsworth et al. [23]. Moderate exercise (“normal/brisk walking” or “walking 3.0 mph at a moderate pace on a level, firm surface”) was assigned a score of 3.5 METs/hour. Wolf et al. found a correlation of α = 0.79 between this assessment criterion and daily diaries kept over a 1-week period [24]. We applied a recent federal guideline that recommends 150 min/week of moderate exercise (e.g., brisk walking for a total of 8.75 METs/week) as sufficient to provide significant medical benefit for US adults with and without chronic illnesses [25].
-
3.
Post-bariatric surgery lifestyle distress was assessed by the Lifestyle Distress Questionnaire (LDQ) [21] This 20-item scale assesses post-surgical treatment regimen distress. The LDQ assesses a range of fears, frustrations, and concerns experienced over the previous week. For example, “Burdened by the constant effort needed to maintain eating and lifestyle changes,” “Annoyed by the need to exercise to lose weight,” and “Sad that I have to give up comfort foods for those times when I am bored, unhappy, or stressed.” LDQ items have a Likert scale format ranging from 0 (“no problem”), 1 (“minor problem”), 2 (“moderate problem”), 3 (“somewhat serious problem”), to 4 (“severe problem”). The LDQ total raw score is converted to a 0–100 range with higher scores indicating higher distress. Internal reliability for the LDQ is α = 0.92, and 2-week test–retest reliability (ICC) is 0.86 [21].
-
4.
Depression was assessed by the Patient Health Questionnaire (PHQ) [26], a widely used nine-item self-report questionnaire assessing symptoms of depression in which participants are asked to rate how they felt in the previous 2 weeks. Each question is scored 0 to 3 (0 = not at all, 1 = several days, 2 = more than half the days, and 3 = nearly everyday) with a scale range of 0 to 27. The nine PHQ items and scoring reflect standard criteria for major depressive disorder. Studies have provided empirical support for its validity and reliability. Sound internal consistency has been reported (α = 0.79–0.89), and there is strong evidence of its criterion, construct, and external validity [27].
-
5.
Perceived benefits of weight loss were assessed with the Perceived Benefits Questionnaire (PBQ) [21]. This 20-item scale assesses a range of perceived benefits including daily functioning and self-perceptions related to post-surgical weight loss. For example, PBQ items include “My self confidence has improved,” “I am happier with my body shape,” “My medications have been reduced by my doctor.” PBQ benefit items have a Likert scale format ranging from 1 (“not at all”), 2 (“a little”), to 3 (“a lot”). The PBQ total raw score was converted to a 0–100 range with higher scores indicating higher benefits. Internal reliability for the PBQ is α = 0.78, and 2-week test–retest reliability (ICC) is 0.64 [21].
-
6.
Binge eating was assessed using an unpublished two-item scale based on key components of Diagnostic and Statistical Manual-IV R criteria [28]. A binge was defined as: “Have you had any episodes of uncontrolled binge eating over the past few months? An eating binge is when you quickly eat a large amount of food, you keep eating until you are uncomfortably full, and you felt guilty or ashamed afterwards.” In this study, binges occurring two times a week or more over the past few months were used to define clinically significant binge eating.
-
7.
Alcohol abuse was assessed by the CAGE scale [29, 30] that has been extensively validated to screen for potential alcoholism [31]. A positive case of alcohol abuse required endorsement for two questions: “Do you drink alcohol (beer, wine, or spirits)?” and “Do you drink more than two drinks a day if you are a man or one drink a day if a woman? A drink is defined as one ounce of 100-proof liquor, four ounces of wine, or 12 ounces of beer.” Then at least three of the following four CAGE items must be endorsed: “Have you ever felt you should cut down on your drinking?”, “Have people annoyed you by criticizing your drinking?”, “Have you ever felt bad or guilty about your drinking?”, and “Have you ever had a drink first thing in the morning to steady your nerves or to get rid of a hangover?”. CAGE test scores ≥2 have a sensitivity of 93% and a specificity of 76% for the identification of problem drinkers [31].
-
8.
Post-surgical clinical exam by the WLS clinical staff to check patient medical and dietary status occurred at 5–7 days (for nurse to remove stitches and review post-surgical recovery) and was then scheduled (for separate surgeon and dietitian sessions on the same day) at 2–3 weeks, 6–8 weeks, 3 months, 6 months, 9 months, 12 months, and 18 months, followed by annual visits. The 9- and 18-month visits were excluded if patient progress was considered satisfactory and uneventful by the surgeon. WLS clinical visits were documented separately for the bariatric surgeon and dietitian through the patient electronic billing and electronic medical record system.
-
9.
Support group attendance was tracked by a log book used at each support group meeting during the study follow-up period. The 2-h support groups were conducted at the hospital three times a month (including a women only group) and led by a WLS clinic dietitian who ensured all patients completed a sign-in sheet if attending the session. The attendance record books were stored in a locked cabinet in the WLS for subsequent data abstraction and analysis.
Statistical Analyses
We examined a range of factors identified in the clinical literature as potentially important in predicting post-surgical weight loss outcome following LGB surgery. These factors included pre-surgical and goal weight, post-surgical behavioral adherence, lifestyle-related emotional distress and depression, perceived benefits of weight loss, and frequency of clinical and support group attendance on weight loss outcome at 2–3-year follow-up. Demographic, clinical, and questionnaire data were analyzed as means and standard deviations, medians, or percentages, as appropriate to the scale of measurement. Univariate comparisons of participants vs. non-participants used Student’s t test for continuous variables and Chi-square with Yates’ continuity correction for fourfold tables and Fishers exact test when an expected cell value was less than 5. Pearson or Spearman rank correlations were used to examine associations among continuous variables involving normal or non-normal distributions, respectively. All pre–post comparisons were analyzed using the paired t test. Stepwise linear regression was used to identify variables that predicted %EWL at follow-up. To avoid multicollinearity, modeling was performed in blocks. In the first block, demographic variables were entered. In subsequent blocks, behaviors and psychosocial variables were entered. At each step, variables were tested for collinearity. The t statistic was used (with inclusion and retention criteria set at p = 0.15) to determine significance of individual variables. The Statistical Package for the Social Sciences [32] and the Statistical Analysis System [33] were used for analyses.
Results
At baseline, the initial sample (n = 100) was 85.3% female, with a mean age of 43.8 ± 10.9 years (range 20–68 years). Racial composition was 84% White, 6.8% Black, 9.2% “other”, while ethnicity was 9.5% Hispanic. Education classification was 20.0% high school graduate or lower, 37.3% some college education, and 42.7% college graduate or higher. Employment status was 66.2% in full time work, 14.9% part time, and 19.9% not currently working.
There was a significant (p < 0.01) change in patient marital status reported at follow-up. Specifically, 49.3% of patients were married or had a partner at baseline compared to 60.8% at follow-up, 21.9% were divorced or separated at baseline compared to 20.3% follow-up, and 28.8% were classified as never married at baseline compared to 18.9% at follow-up. Overall, 26.4% of patients reported change in marital status from baseline to follow-up. Two percent of the sample reported smoking behavior at the time of surgery.
Follow-up Assessments
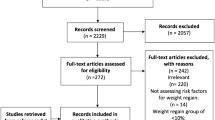
Patients were followed for a mean (±SD) time since surgery of 917.1 ± 96.8 days (approximately 2.5 years) with a range from 2.1 to 3.2 years. Five patients received an LGB surgical revision for staple line breakdown and associated weight gain. These patients had previously undergone gastric bypass by another surgeon, and their staple line dehiscience (i.e., gastro-gastric fistula) was repaired during the revision. Therefore, we excluded these patients from the analyses (Fig. 1). Statistical comparison of participants (n = 75) with non-participants (n = 25, comprising surgical revisions, deaths, refusals, not located) showed that participants were significantly different at baseline in both age (43.8 ± 10.9 vs. 39.0 ± 8.8 years, p < 0.04) and marital status (divorced or separated 21.9% vs. 0%; never married 28.8% vs. 25.0%; married or with partner 49.3% vs. 75.0%, p < 0.03) but not in gender, education, work status, baseline weight or BMI, or goal weight.
At follow-up, mean body weight in pounds dropped from 301.1 (95%CI 288.0–314.1) to 206.4 (95%CI 195.5–217.3) (p < 0.001) representing an average weight loss in pounds of 96.5 (95%CI 88.1–104.9; range 20–223) and a %EWL of 59.1% (95%CI 55.0–63.1) (Table 1). BMI (kg/m2) improved from a mean of 49.8 (95%CI 48.3–51.4) to 34.1 (95%CI 32.7–35.6) (p < 0.001) that represented a %BML change of −31.6% (95%CI −33.7 to −29.4). There was a significant difference between the patient’s baseline goal weight in pounds (mean 162.9, 95%CI 154.5–171.3) and the achieved weight at follow-up (mean difference 43.6 lb, 95%CI 33.4–53.8; p < 0.001). The proportion of patients who achieved the %ELW > 50% criterion was 70.8%.
Mean BSSQ subscale behavioral adherence scores are shown in Table 2. The strongest adherence occurred for protein intake (78.1%), dumping syndrome management (76.5%), and fruit and vegetable intake (71.7%). Lowest behavioral adherence rates were for physical activity (56.2%). Mean self-reported physical activity level in (METs/week) was obtained by clinician interview and use of the NHS-II assessment and improved from a mean of 1.3 ± 4.0 to mean of 14.0 ± 17.8 (t = −5.54, df = 67, p < 0.01). While only 7.3% of participants met national physical activity guidelines at baseline, 51.4% of participants met these criteria at follow-up.
Results of psychosocial assessments (see Table 2) showed that mean post-surgical lifestyle distress (LDQ) was 19.8 (0–100 scale), and mean perceived benefit of weight loss from LGB surgery was 82.7 (0–100 scale), indicating low emotional distress and high perceived weight loss benefit. There were no cases of overt depression at follow-up, but 32% were taking antidepressant medication to maintain this level of symptomatic control. Only 1.3% met the criteria for alcohol abuse, 12.0% reported current binge eating, and 12% were current smokers. Patient attendance at scheduled post-surgical follow-up visits was a mean of 4.8 ± 2.7 visits for bariatric surgeon (range 1–14), 4.8 ± 2.4 visits for dietician, and support group attendance within 2 years of surgery was a mean of 6.2 ± 7.9.
Laboratory findings for nutritional status (percentage out of normal range) shown in Table 3 were as follows: vitamin A (all in range), thiamin (25.8% low), vitamin B12 (16.4% high), calcium (1.5% low), vitamin D (19.4% low), ferritin (32.8% low), folic acid (all in range), iron (9.0% low), IBC-saturated (30.3% low), and IBC-unsaturated (7.6% high). Table 4 shows the prevalence of MO-related co-morbidities identified at follow-up. Diabetes was present for 14.9% (HbA1c ≥ 6%), while another 13.4% were under control with blood glucose lowering medications (see Table 4). For hypertension, no patients were actively hypertensive (≥140/90 mm Hg), but another 52.8% required medications to achieve adequate control. For hypercholesterolemia, only 2.8% were not controlled (LDL > 100 mg/dL), but another 52.8% required medications to achieve control.
The results of stepwise regression analyses used to predict weight loss at follow-up are shown in Table 5. Variables tested in block 1 were age, education, marital status, ethnicity, and pre-surgical weight. In block 2, the seven BSSQ post-surgical self-management subscales were tested. In block 3, LDQ (lifestyle emotional distress), PHQ (depression), the PBQ (benefits of weight loss), frequency of clinic and support group attendance, and duration in months since surgery were tested. Physical activity was also examined using the NHS-II PA interview to complement use of the physical activity subscale of the BSSQ questionnaire (Table 5). In the final regression model, the only significant predictors of %EWL were physical activity and baseline weight accounting for 13% of total variance (R 2 = 0.13).
Discussion
The primary goal of this follow-up study of 100 RYGB gastric bypass patients was to estimate weight loss and clinical outcomes related to cardiovascular risk at 2–3 years from surgery when weight loss is thought to plateau for most patients [28]. We also examined a range of potential clinical, behavioral, and psychosocial mechanisms of weight loss as these have not been well studied to date.
We aimed to minimize patient loss to follow-up given that our recent literature review identified this as a significant research issue [34]. Specifically, we found a mean of 71% of patients had been lost to follow-up for studies with 2-year endpoints and 69% for those with 3-year endpoints [5]. In this study, 75% of the original cohort took part, thus providing a substantially improved estimate of weight loss in LGB at 2–3 years compared to prior research. Comparison of study participants and non-participants showed the latter to be younger and more likely to be married but otherwise similar in profile, reducing concerns of systematic bias in our findings.
The results showed our LGB surgery patients had a percent excess weight loss of 59.1% at a mean of 2.5 years after surgery. This estimate is very similar to the 61.6% estimate found from a meta-analysis of 22 open and laparoscopic gastric bypass studies conducted by Buchwald [4] and the 62.2% estimate reported in our subsequent meta-analysis of 29 laparoscopic gastric bypass studies [5].
Rates of current diabetes (14.9%), hypertension (0%), and hyperlipidemia (2.8%), were found among our patient cohort to be much lower at follow-up compared to rates reported earlier for MO patients in the general population (i.e., 44.6%, 21.0%, and 47.0%, respectively) [34]. It is notable that many of our patients were able to achieve good metabolic control but only with the continued use of some medications. Overall, the loss of an average of 59% of excess weight at 2–3 years post-surgery, in combination with continued medication use for a subset of patients, had reduced the prevalence of metabolic syndrome to 10.6% compared to 52% reported earlier for bariatric surgery patients and 25% for the adult US population [35]. Thus, this study confirms that LGB confers a very important reduction in overall cardiovascular risk for MO patients. While smoking rates were only 2% prior to surgery as a result of compliance with surgery criterion for eligibility and use of pre-surgical urine cotinine testing, some patients (12%) had resumed this high-risk behavior for not only cardiovascular risk but also marginal ulcer and anastomotic stricture.
Unexpectedly, our examination of behavioral mechanisms of weight loss at follow-up showed self-reported adherence to clinically recommended eating behaviors (e.g., eating small, frequent meals, slow chewing of food until a pureed consistency, stopping at first signs of fullness, etc.), fluid intake behaviors (e.g., avoiding fluids for 30 min before and after meals, hydrating well with frequent small sips of water throughout the day), as well as management of dumping syndrome, protein intake, and fruit and vegetable intake were not related to weight loss outcome. A priori, this would be expected to strongly influence patient experience of pouch fullness and/or satiety and thus influence overall calorie intake and weight loss. However, the non-significant results found here for the influence of eating style and fluid intake on weight loss were similar to those we reported earlier in a cross-sectional study involving patients attending post-surgical support groups using the same self-report questionnaire [21]. Patient hunger and satiety were not evaluated in this study for their role in weight loss outcome, although they are observed clinically to be dramatically changed following gastric bypass surgery. These factors could be explored in future research to help us understand post-surgical eating behavior and weight loss and our non-significant findings for food intake. It is possible that the focus in our behavioral assessment questionnaire on optimal self-management behaviors is narrow and overlooks important eating pathology. However, we did assess binge eating in this study, and this was present among 12% at follow-up but was not related to weight loss. Future research might include a closer examination of maladaptive eating behaviors commonly seen before surgery that could have re-emerged for some patients such as grazing, night eating, intake of high calorie drinks, or emotional eating unrelated to hunger with careful attention to methodological rigor in these assessments.
The multiple regression analysis of post-surgical behaviors showed that physical activity was the only post-surgical behavior associated with weight loss at follow-up, with greater activity associated with improved weight loss. Combining with pre-surgical patient weight (also positively associated), this accounted for 13% of total weight loss variance. We assessed physical activity using both a questionnaire and interview format with similar findings. This result for the association of exercise to improved weight loss is similar to that for our earlier study focused on post-surgical support group patients [21] and those reported in other cross-sectional studies [36–38]. For example, Hatoum et al. found baseline weight and physical activity to be the strongest predictor of %EWL 1 year post-surgery [36]. Given that self-report may be associated with inaccurate or biased reporting, these findings could be considered useful for hypothesis generation at this nascent stage of research in this area. Prospective studies involving objective physical activity assessments such as pedometers, accelerometers, heart rate monitors, indirect calorimetry, or the doubly labeled water technique, validated for MO populations, could greatly advance our knowledge. It is encouraging that while our patients were mostly sedentary prior to surgery (only 7.3% reported exercising at a level that meets national guidelines expected to provide medical benefit), at follow-up, this proportion was 50%. It was not specifically examined in this study whether the loss of an average of 96.5 lb significantly reduced symptoms of breathlessness, joint pain, fatigue, etc., that made higher levels of exercise more achievable, but overall the perceived benefit from weight loss was high (around 80%), including endorsement of significant improvement in physical mobility (walking, taking the stairs, lifting, and carrying).
Interestingly, given the emphasis placed in pre-surgical screening of bariatric surgery candidates on the need for significant patient motivation and behavioral adjustment following surgery, emotional distress associated with the new lifestyle was low (around 20%). These findings are identical to those we found for 200 consecutive patients attending an RGB support group [21] and suggests that post-surgical lifestyle is not emotionally burdensome. The additional finding that 26% of patients reported a change in marital status (i.e., now divorced, remarried, or once single but now married), within the short 2–3-year period following surgery was surprising. Prior reports have produced mixed findings on this issue in terms of marital status and its association with weight loss [37, 38]. More systematic investigation involving in-depth assessments and clinical interviews of patients and partners is needed to explore this issue further.
We expected, based on the clinical literature [13], that frequency of patient attendance to clinical follow-up and level of attendance to hospital-based patient support groups would predict weight loss success. However, while we found mean frequency of clinic follow-up was around five visits over 2 years (out of a total of eight scheduled clinic visits) and support group attendance at a mean of six sessions (with monthly groups available to patients excluding holiday breaks over the duration of the 2–3-year follow-up period), these factors were not significantly related to weight loss outcome.
Finally, given that LGB involves a malabsorptive mechanism to reduce calorie intake and partial gastric and duodenal exclusion [39], we were interested to examine patient-reported adherence to prescribed post-surgical vitamin and mineral supplements and examine related blood chemistry findings. Mean reported supplement adherence to daily multivitamin, calcium, and B-complex vitamin supplements was overall only 57.6%, and suboptimal blood chemistry levels were found for thiamine (25% of patients), ferritin (32%), vitamin D (19%), and hematocrit (27%). Most concerning were the findings for thiamine as this deficiency is generally considered rare and expected only when the patient has nausea and vomiting within 3 months post-surgery and/or shows signs of neurologic problems. The clinical significance of the blood chemistry findings and their relationship to vomiting or food intake were not examined in this study but the poor patient supplement adherence findings reinforce concerns raised in prior reviews and the need for clinicians to stress the importance of patient adherence to prescribed vitamin and mineral supplements for the patient’s lifetime to avoid the possibility of deficiency conditions [40].
There were a number of limitations to the study design that limit the generalizability of the findings and suggest caution in interpretation of our findings beyond the issues raised above. Specifically, clinical factors related to cardiovascular risk reduction (diabetes, hypertension, and hypercholesterolemia) examined at follow-up were not collected at baseline, although we have provided baseline prevalence estimates for other MO patient groups to help with the interpretation of our follow-up findings. Second, we did not prospectively gather data on weight loss or clinical and behavioral findings at repeated intervals from surgery to follow-up that may have identified important trends, such as weight loss fluctuations or significant variability in adherence to physical activity and other target lifestyle behaviors over time. Third, while we assessed recommended patient eating behaviors expected to influence weight loss as well as binge eating, we did not examine other related factors that could have provided a fuller picture, such as hunger, satiety, and actual food intake in terms of calories ingested and nutritional quality. Addictive behaviors have been suggested to emerge following LGB as part of a transference of patient focus from food to other gratifying behaviors such as gambling compulsion and alcohol and substance abuse [41]. We did not specifically explore this issue, but interestingly found only 1.3% of patients were positive for alcohol abuse using a widely used clinical screening tool.
In summary, this study identified a mean percent excess weight loss of 59.1% for a cohort LGB patients assessed 2–3 years after surgery. A key feature of this study was its focus on study quality in terms of patient follow-up rate and its focus on potential mediators of weight loss. We found significant weight loss at follow-up that was associated with greatly reduced cardiovascular co-morbidity status compared to US population benchmarks. Overall, a pattern of positive psychosocial functioning was reported by patients, although adherence to mandated daily multivitamin and mineral supplements to avoid potential nutritional deficiency conditions was of concern. Related laboratory findings were notable for thiamine (B1), in particular. It was surprising that recommended eating and fluid intake behaviors as well as frequency of clinic and support group attendance were not predictors of weight loss. However, level of physical activity based on patient self-report of minutes of moderate exercise emerged as a potentially important factor in explaining weight loss variance. Future studies could advance our nascent knowledge in this area by applying objective exercise assessment strategies shown to be feasible and valid for MO populations to further explore the role of physical activity and exercise in weight loss following RGB.
References
Buchwald H. Obesity co-morbidities. In: Buchwald H, Cowan GSM, Pories WJ, editors. Surgical management of obesity. Philadelphia: Saunders; 2007. p. 37–44.
Sturm R. Increases in morbid obesity in the USA: 2000–2005. Public Health. 2007;121:492–6.
American Society for Metabolic and Bariatric Surgery (ASMBS) Fact Sheet. http://www.asbs.org/Newsite07/media/fact-sheet1_bariatric-surgery.pdf. Accessed 10 Apr 2009
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37.
Garb J, Welch GW, Zagarins S, et al. Bariatric surgery for the treatment of morbid obesity: a meta-analysis of weight loss outcomes for laparoscopic adjustable gastric banding and laparoscopic gastric bypass. Obes Surg. 2009;19(10):1447–55.
Oria HE. Long term follow-up and evaluation of results in bariatric surgery. In: Buchwald H, Cowan GSM, Pories WJ, editors. Surgical management of obesity. Philadelphia: Saunders; 2007. p. 345–56.
Andrews G, LeMont D, Myers S. Caring for the surgical weight loss patient. Sierra Madre: Wheat Field; 2003.
McMahon M, Sarr M, Clark M, et al. Clinical management after bariatric surgery: value of a multidisciplinary team. Mayo Clin Proc. 2006;81:S34–45.
Saunders R. Grazing: a high risk behavior. Obes Surg. 2004;14:98–102.
Poole N, AlAtar A, Kuhanendran D. Compliance with surgical after-care following bariatric surgery for morbid obesity: a retrospective study. Obes Surg. 2005;15:261–5.
Silver H, Torquati A, Jensen G. Weight, dietary, and physical activity behaviors two years after gastric bypass. Obes Surg. 2006;16:859–64.
Colles S, Dixon J. Night eating syndrome: impact on bariatric surgery. Obes Surg. 2007;16:811–20.
DiRocco JD, Halverson JD, Planer J, et al. In: Buchwald H, Cowan GSM, Pories WJ, editors. Surgical management of obesity. Philadelphia: Saunders; 2007.
Santri H, Alverdy JC, Prachand VN. Patient selection for bariatric surgery. In: Buchwald H, Cowan GSM, Pories WJ, editors. Surgical management of obesity. Philadelphia: Saunders; 2007.
Magro DO, Geloneze B, Delfini R, et al. Long-term weight regain after gastric bypass: a 5-year prospective study. Obes Surg. 2008;6(18):648–51. 2006;16:811-820.
Welch G, Wesolowski C, Piepul P, et al. Perioperative symptoms following gastric bypass surgery. Bariatric Nursing and Surgical Patient Care. 2008;3(2):159–63.
Deitel M, Gawdat K, Melissas J. Reporting weight loss 2007. Obes Surg. 2007;17:565–8.
Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). National Cholesterol Education Program. National Institutes of Health Publication No. 01-3670, 2001.
Longitudinal Assessment of Bariatric Surgery. National Institutes of Health. http://www.edc.gsph.pitt.edu/labs/Public/
Kincaid JP, Fishburne, RP, Rogers RL, et al. Derivation of new readability formulas (Automated Readability Index, Fog Count and Flesch Reading Ease Formula) for Navy enlisted personnel, Research Branch Report 8-75, Millington, TN: Naval Technical Training, U. S. Naval Air Station, Memphis; 1975.
Welch G, Wesolowski C, Piepul B, et al. Physical activity predicts weight loss following gastric bypass surgery: findings from a support group survey. Obes Surg. 2008;18(5):517–24.
Weinstein AR, Sesso HD, Lee IM, et al. Relationship of physical activity vs body mass index with type 2 diabetes in women. JAMA. 2004;292(10):1188–94.
Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9):S498–504.
Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–9.
Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: U.S. Department of Health and Human Services, 2008.
Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann. 2002;35:509–21.
The Macarthur Initiative of depression and primary care. http://www.depression-primarycare.org
American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders, DSM-IV. Washington, DC, American Psychological Association, 2000.
Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905–7.
Kitchens JM. Does this patient have an alcohol problem? JAMA. 1994;272(22):1782–7.
Bernadt MW. Comparison of questionnaire and laboratory tests in the detection of excessive drinking and alcoholism. Lancet. 1982;6(8267):325–8.
SPSS. Statistical Package for the Social Sciences (SPSS). 10.0 Version. Chicago: SPSS Inc; 1999.
SAS. Statistical Analysis System (SAS). 9.1 Version. Cary: SAS Inc; 2005.
Mondolfi RN, Jones TM, Hyre AD, Raggi P, Muntner P. Comparison of percent of United States adults weighing>or=300 pounds (136 kilograms) in three time periods and comparison of five atherosclerotic risk factors for those weighing>or=300 pounds to those<300 pounds. Am J Cardiol. 2007;100(11):1651–3.
Lee WJ, Huang M, Wang W, et al. Effects of obesity surgery on the metabolic syndrome. Arch Surg. 2004;139:1088–92.
Hatoum IJ, Stein HK, Merrifield BF, et al. Capacity for physical activity predicts weight loss after Roux-en-Y gastric bypass. Obesity. 2009;17(1):92–9.
Lutfi R, Torquati A, Sekhar N. Predictors of success after laparoscopic gastric bypass: a multivariate analysis of socioeconomic factors. Surg Endosc. 2006;20(6):864–7.
van Hout GC, Verschure SK. Psychosocial predictors of success following bariatric surgery. Obes Surg. 2005;15(4):552–60.
Dixon JB, O’Brien PE. Nutritional outcomes of bariatric surgery. In: Buchwald H, Cowan GSM, Pories WJ, editors. Surgical management of obesity. Philadelphia: Saunders; 2007. p. 357–64.
Marcus J, Symmonds R, Rodriguez J, et al. Noncompliance with behavioral recommendations following bariatric surgery. Obes Surg. 2005;15(4):546–51.
Odom J, Zalesin KC, Washington TL, et al. Behavioral predictors of weight regain after bariatric surgery. Obes Surg. 2009. doi:10.1007/s11695-009-9895-6.
Author information
Authors and Affiliations
Corresponding author
Appendix: Bariatric Surgery Self-management Questionnaire (BSSQ) Subscales and Item Content
Appendix: Bariatric Surgery Self-management Questionnaire (BSSQ) Subscales and Item Content
Eating behaviors (EB):
-
1.
I ate a minimum of 5 mini meals or snacks during the day
-
2.
I ate slowly, putting my utensils or food down between bites
-
3.
It took about 20-30 minutes for me to eat my meals
-
4.
I chewed my food until it was a pureed consistency like baby food
-
5.
I used a bread and butter plate or dessert plate instead of a regular- sized plate for my meals
-
6.
I checked for feeling of a feeling of fullness after every bite
-
7.
I stopped eating immediately if I had any feelings of fullness or discomfort
-
8.
I used a baby spoon, fork, and knife instead of regular sized ones
Fluid intake (FI):
-
9.
I drank 48 ounces (six 8 oz glasses) or more of fluids during the day
-
10.
I only drank water, sugar-free beverages, skim milk, or 1% milk
-
11.
I sipped drinks slowly putting my drink down between sips
-
12.
I avoided using a straw to drink
-
13.
I carried a suitable drink with me at all times
-
14.
I did not rely on feeling thirsty as a signal to drink
-
15.
I checked my urine through the day to make sure it was pale yellow-to-clear in color showing good fluid intake
-
16.
I drank 30 minutes before my meal and waited until 30 minutes after my meal so that I separated my fluids from my solid foods
Physical activity (PA):
-
17.
I got 30-60 minutes of exercise 5 days or more in the past week (e.g., walking, exercise equipment at home, health club, class, etc)
-
18.
I built some exercise into my daily routines (I took the stairs, walked around the supermarket or mall before shopping, etc.)
-
19.
I built some weight training into my exercise program (hand weights, climbing stairs, weight machines, etc)
Dumping syndrome management (DSM):
-
20.
I read nutrition fact panels on food labels to look for high levels of sugar
-
21.
I avoided foods and beverages with 15 grams (3 tsp) of sugar or more a serving
-
22.
I avoided foods and beverages with sugar listed as one of the first three ingredients (glucose, maltose, dextrose, fructose, honey, molasses, corn syrup, brown sugar, cane sugar, confectionary sugar)
-
23.
I avoided sugar alcohols (mannitol, sorbitol, xylitol, lactitol) by looking at food labels as these cause cramping and diarrhea
Supplement Intake (SI):
-
24.
I took a multi-vitamin with minerals tablet every day
-
25.
I took 1000 to 1500 mg of calcium citrate or calcium carbonate with vitamin D every day
-
26.
I took a B-complex vitamin supplement every day
-
27.
I took my vitamin/calcium pills 4 hours or more apart to maximize absorption
Fruits, vegetables, and whole grain intake (FVW):
-
28.
I ate at least 5 fruits and vegetables every day
-
29.
I mostly chose whole grain breads, cereals, and crackers
-
30.
I mostly chose brightly colored fruits and vegetables (yellow, green, red, orange, blue, purple)
Protein Intake (PI):
-
31.
I ate 60-80 grams (2-3 oz) of protein every day (fish, eggs, chicken, turkey, beef, pork, ham, milk, peanut butter, beans, soy, tofu, lentils, cheese, nuts, yogurt, skim or 1% milk, or low sugar protein bars and shakes)
-
32.
I ate the protein on my plate first during meals and snacks
-
33.
I read food labels and chose the foods highest in protein and lowest in sugar
Rights and permissions
About this article
Cite this article
Welch, G., Wesolowski, C., Zagarins, S. et al. Evaluation of Clinical Outcomes for Gastric Bypass Surgery: Results from a Comprehensive Follow-up Study. OBES SURG 21, 18–28 (2011). https://doi.org/10.1007/s11695-009-0069-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-009-0069-3