Abstract
Background
Patient adherence to warfarin may influence anticoagulation control; yet, adherence among warfarin users has not been rigorously studied.
Objective
Our goal was to quantify warfarin adherence over time and to compare electronic medication event monitoring systems (MEMS) cap measurements with both self-report and clinician assessment of patient adherence.
Design
We performed a prospective cohort study of warfarin users at 3 Pennsylvania-based anticoagulation clinics and assessed pill-taking behaviors using MEMS caps, patient reports, and clinician assessments.
Results
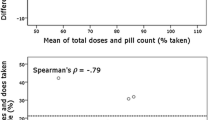
Among 145 participants, the mean percent of days of nonadherence by MEMS was 21.8% (standard deviation±21.1%). Participants were about 6 times more likely to take too few pills than to take extra pills (18.8 vs. 3.3%). Adherence changed over time, initially worsening over the first 6 months of monitoring, which was followed by improvement beyond 6 months. Although clinicians were statistically better than chance at correctly labeling a participant’s adherence (odds ratio = 2.05, p = 0.015), their estimates often did not correlate with MEMS-cap data; clinicians judged participants to be “adherent” at 82.8% of visits that were categorized as moderately nonadherent using MEMS-cap data (≥20% nonadherence days). Similarly, at visits when participants were moderately nonadherent by MEMS, they self-reported perfect adherence 77.9% of the time.
Conclusions
These results suggest that patients may benefit from adherence counseling even when they claim to be taking their warfarin or the clinician feels they are doing so, particularly several months into their course of therapy.


Similar content being viewed by others
References
Chiquette E, Amato MG, Bussey HI. Comparison of an anticoagulation clinic with usual medical care: anticoagulation control, patient outcomes, and health care costs. Arch Intern Med. 1998;158:1641–7.
Palareti G, Leali N, Coccheri S, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet. 1996;348:423–8.
Kutner M, Nixon G, Silverstone F. Physicians’ attitudes toward oral anticoagulants and antiplatelet agents for stroke prevention in elderly patients with atrial fibrillation. Arch Intern Med. 1991;151:1950–3.
Bush D, Tayback M. Anticoagulation for nonvalvular atrial fibrillation: effects of type of practice on physicians’ self-reported behavior. Am J Med. 1998;104:148–51.
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97.
Kimmel SE, Chen Z, Price M, et al. The influence of patient adherence on anticoagulation control with warfarin: results from the International Normalized Ratio Adherence and Genetics (IN-RANGE) Study. Arch Intern Med. 2007;167:229–35.
Howard AF, Frewin DB, Leonello PP, Taylor WB. Compliance with anticoagulant drug therapy: a study on patients with prosthetic heart valves. Med J Aust. 1981;2:274–6.
Van der Meer FJ, Briet E, Vandenbroucke JP, et al. The role of compliance as a cause of instability in oral anticoagulant therapy. Br J Haematol. 1997;98:893–900.
Laporte S, Quenet S, Buchmuller-Cordier A, et al. Compliance and stability of INR of two oral anticoagulants with different half-lives: a randomised trial. Thromb Haemost. 2003;89:458–67.
Butler JA, Peveler RC, Roderick P, et al. Measuring compliance with drug regimens after renal transplantation: comparison of self-report and clinician rating with electronic monitoring. Transplantation. 2004;77:786–9.
Byerly M, Fisher R, Whatley K, et al. A comparison of electronic monitoring vs. clinician rating of antipsychotic adherence in outpatients with schizophrenia. Psychiatry Res. 2005;133:129–33.
Gross R, Bilker WB, Friedman HM, Coyne JC, Strom BL. Provider inaccuracy in assessing adherence and outcomes with newly initiated antiretroviral therapy. AIDS. 2002;16(13):1835–7.
Pullar T, Kumar S, Tindall H, Feely M. Time to stop counting the tablets? Clin Pharmacol Ther. 1989;46:163–8.
Cramer JA, Mattson RH, Prevey ML, et al. How often is medication taken as prescribed? A novel assessment technique. JAMA. 1989;261:3273–7.
Urquhart J. Role of patient compliance in clinical pharmacokinetics. A review of recent research. Clin Pharmacokinet. 1994;27:202–15.
Urquhart J. The electronic medication event monitor. Lessons for pharmacotherapy. Clin Pharmacokinet. 1997;32:345–56.
Wilson SJ, Wells PS, Kovacs MJ, et al. Comparing the quality of oral anticoagulant management by anticoagulation clinics and by family physicians: a randomized controlled trial. CMAJ. 2003;169:293–8.
Gallus AS. Towards the safer use of warfarin: an overview. J Qual Clin Pract. 1999;19:55–9.
Ansell JE, Buttaro ML, Thomas OV, Knowlton CH. Consensus guidelines for coordinated outpatient oral anticoagulation therapy management. Anticoagulation Guidelines Task Force. Ann Pharmacother. 1997;31:604–15.
Acknowledgements
This study was supported by grants from the NIH (R01-HL66176) and Agency for Healthcare Research and Quality (P01-HS11530). Dr. Kimmel is supported by P20RR020741. The authors would like to thank Anita Hill, BS; Kiasha Huling, BA; Linda Bowman, BS, RN; Mitchell Laskin, RPh; Mabel Chin, PharmD; and Francis Herrmann, BS, RPh, for their dedicated field work; Joseph Gascho, MD, for serving as the site investigator at the Hershey Medical Center; and Sandy Barile for editorial assistance.
Conflict of Interest
Dr. Kimmel has received research funding from GlaxoSmithKline and Pfizer, and has served as a consultant to Bayer, GlaxoSmithKline, Bristol-Meyers Squibb, and Pfizer, all unrelated to warfarin. Dr. Gross has received research funding from Bristol-Myers Squibb, unrelated to warfarin. Dr. Samaha has received grant support from Merck, Sharpe and Dome; Abbott; Kos Pharmaceuticals; and Aegerion, all unrelated to warfarin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parker, C.S., Chen, Z., Price, M. et al. Adherence to Warfarin Assessed by Electronic Pill Caps, Clinician Assessment, and Patient Reports: Results from the IN-RANGE Study. J GEN INTERN MED 22, 1254–1259 (2007). https://doi.org/10.1007/s11606-007-0233-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-007-0233-1