Abstract
Purpose
Research studies that measure health-related quality of life (HRQOL) in both children and adults and longitudinal studies that follow children into adulthood need measures that can be compared across these age groups. This study links the PROMIS pediatric and adult emotional distress measures using data from participants with diverse health conditions and disabilities.
Methods
Analyses were conducted and compared in two separate samples to confirm the stability of results. One sample (n = 874) included individuals aged 14–20 years with special health care needs and who require health services. The other sample (n = 641) included individuals aged 14–25 years who have a physical or cognitive disability. Participants completed both PROMIS pediatric and adult measures. Item response theory-based scores were linked using the linear approximation to calibrated projection.
Results
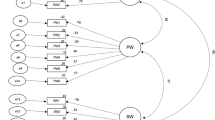
The estimated latent-variable correlation between pediatric and adult PROMIS measures ranged from 0.87 to 0.94. Regression coefficients β 0 (intercept) and β 1 (slope), and mean squared error are provided to transform scores from the pediatric to the adult measures, and vice versa.
Conclusions
This study used a relatively new linking method, calibrated projection, to link PROMIS pediatric and adult measure scores, thus expanding the use of PROMIS measures to research that includes both populations.

Similar content being viewed by others
References
DeWalt, D. A., Rothrock, N., Yount, S., & Stone, A. A. (2007). Evaluation of item candidates: The PROMIS qualitative item review. Medical Care, 45(5 Suppl 1), S12–S21. doi:10.1097/01.mlr.0000254567.79743.e200005650-200705001-00003.
Irwin, D. E., Varni, J. W., Yeatts, K., & DeWalt, D. A. (2009). Cognitive interviewing methodology in the development of a pediatric item bank: A patient reported outcomes measurement information system (PROMIS) study. Health and Quality of Life Outcomes, 7, 3. doi:10.1186/1477-7525-7-3.
Dorans, N. J. (2007). Linking scores from multiple health outcome instruments. Quality of Life Research, 16(Suppl 1), 85–94. doi:10.1007/s11136-006-9155-3.
Bjorner, J. B., Chang, C. H., Thissen, D., & Reeve, B. B. (2007). Developing tailored instruments: item banking and computerized adaptive assessment. Quality of Life Research, 16(Suppl 1), 95–108. doi:10.1007/s11136-007-9168-6.
Thissen, D., Reeve, B. B., Bjorner, J. B., & Chang, C. H. (2007). Methodological issues for building item banks and computerized adaptive scales. Quality of Life Research, 16(Suppl 1), 109–119. doi:10.1007/s11136-007-9169-5.
McHorney, C. A., & Cohen, A. S. (2000). Equating health status measures with item response theory: Illustrations with functional status items. Medical Care, 38(9 Suppl), II43–II59. doi:10.1097/00005650-200009002-00008.
Haley, S. M., Ni, P., Lai, J. S., Tian, F., Coster, W. J., Jette, A. M., et al. (2011). Linking the activity measure for post acute care and the quality of life outcomes in neurological disorders. Archives of Physical Medicine and Rehabilitation, 92(10 Suppl), S37–S43. doi:10.1016/j.apmr.2011.01.026.
Masse, L. C., Allen, D., Wilson, M., & Williams, G. (2006). Introducing equating methodologies to compare test scores from two different self-regulation scales. Health Education Research, 21(Suppl 1), i110–i120. doi:10.1093/her/cyl088.
Orlando, M., Sherbourne, C. D., & Thissen, D. (2000). Summed-score linking using item response theory: Application to depression measurement. Psychological Assessment, 12(3), 354–359.
Tian, F., Ni, P., Mulcahey, M. J., Hambleton, R. K., Tulsky, D., Haley, S. M., & Jette, A. M. (2014). Tracking functional status across the spinal cord injury lifespan: Linking pediatric and adult patient-reported outcome scores. Archives of Physical Medicine and Rehabilitation, 95(11), 2078 e2015–2085 e2015. doi:10.1016/j.apmr.2014.05.023.
Fisher, W. P, Jr, Eubanks, R. L., & Marier, R. L. (1997). Equating the MOS SF36 and the LSU HSI Physical Functioning Scales. Journal of Outcome Measurement, 1(4), 329–362.
Bond, T. G., & Fox, C. M. (2007). Applying the Rasch model: Fundamental measurement in the human sciences. Mahwah, NJ: Lawrence Erlbaum Associates.
Holzner, B., Bode, R. K., Hahn, E. A., Cella, D., Kopp, M., Sperner-Unterweger, B., & Kemmler, G. (2006). Equating EORTC QLQ-C30 and FACT-G scores and its use in oncological research. European Journal of Cancer, 42(18), 3169–3177. doi:10.1016/j.ejca.2006.08.016.
Wahl, I., Lowe, B., Bjorner, J. B., Fischer, F., Langs, G., Voderholzer, U., et al. (2014). Standardization of depression measurement: A common metric was developed for 11 self-report depression measures. Journal of Clinical Epidemiology, 67(1), 73–86. doi:10.1016/j.jclinepi.2013.04.019.
Huang, I. C., Wu, A. W., & Frangakis, C. (2006). Do the SF-36 and WHOQOL-BREF measure the same constructs? Quality of Life Research, 15(1), 15–24. doi:10.1007/s11136-005-8486-9.
Thissen, D., Liu, Y., Magnus, B., & Quinn, H. (2015). Extending the use of multidimensional IRT calibration as projection: Many-to-one linking and linear computation of projected scores. In L. A. van der Ark, D. M. Bolt, W-C. Wang, J. A. Douglas & S-M. Chow (Eds.), Quantitative Psychology Research: The 79th annual meeting of the psychometric society, Madison, Wisconsin, 2014 (pp. 1–16). New York: Springer.
Irwin, D. E., Stucky, B., Langer, M. M., Thissen, D., Dewitt, E. M., Lai, J. S., et al. (2010). An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Quality of Life Research, 19(4), 595–607. doi:10.1007/s11136-010-9619-3.
Irwin, D. E., Stucky, B. D., Langer, M. M., Thissen, D., Dewitt, E. M., Lai, J. S., et al. (2012). PROMIS Pediatric Anger Scale: An item response theory analysis. Quality of Life Research, 21(4), 697–706. doi:10.1007/s11136-011-9969-5.
Pilkonis, P. A., Choi, S. W., Reise, S. P., Stover, A. M., Riley, W. T., & Cella, D. (2011). Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): Depression, anxiety, and anger. Assessment, 18(3), 263–283. doi:10.1177/1073191111411667.
McPherson, M., Arango, P., Fox, H., Lauver, C., McManus, M., Newacheck, P. W., et al. (1998). A new definition of children with special health care needs. Pediatrics, 102(1 Pt 1), 137–140. doi:10.1542/peds.102.1.137.
Neff, J. M., Sharp, V. L., Muldoon, J., Graham, J., Popalisky, J., & Gay, J. C. (2002). Identifying and classifying children with chronic conditions using administrative data with the clinical risk group classification system. Ambulatory Pediatrics, 2(1), 71–79. doi:10.1367/1539-4409(2002)002<0071:iaccwc>2.0.co;2.
Bethell, C. D., Read, D., Stein, R. E., Blumberg, S. J., Wells, N., & Newacheck, P. W. (2002). Identifying children with special health care needs: development and evaluation of a short screening instrument. Ambulatory Pediatrics, 2(1), 38–48. doi:10.1367/1539-4409(2002)002<0038:icwshc>2.0.co;2.
Gershon, R., Rothrock, N. E., Hanrahan, R. T., Jansky, L. J., Harniss, M., & Riley, W. (2010). The development of a clinical outcomes survey research application: Assessment Center. Quality of Life Research, 19(5), 677–685. doi:10.1007/s11136-010-9634-4.
Irwin, D. E., Stucky, B. D., Thissen, D., Dewitt, E. M., Lai, J. S., Yeatts, K., et al. (2010). Sampling plan and patient characteristics of the PROMIS pediatrics large-scale survey. Quality of Life Research, 19(4), 585–594. doi:10.1007/s11136-010-9618-4.
Dorans, N. J. (2004). Equating, concordance, and expectation. Applied Psychological Measurement, 28, 227–246. doi:10.1177/0146621604265031.
Dorans, N. J., & Walker, M. E. (2007). Sizing up linkages. In N. J. Dorans, M. Pommerich, & P. W. Hollands (Eds.), Linking and aligning scores and scales (pp. 179–198). New York: Springer. doi:10.1007/978-0-387-49771-6_10.
Dorans, N. J., & Holland, P. W. (2000). Population invariance and the equatability of tests: Basic theory and the linear case. Journal of Educational Measurement, 37(4), 281–306. doi:10.1002/j.2333-8504.2000.tb01842.x.
Cai, L., Thissen, D., & du Toit, S. H. C. (2011). IRTPRO for windows. Lincolnwood, IL: Scientific Software International.
Samejima, F. (1997). Graded response model. In W. J. van der Liden & R. K. Hambleton (Eds.), Handbook of modern item response theory (pp. 85–100). New York: Springer. doi:10.1007/978-1-4757-2691-6_5.
Samejima, F. (1969). Estimation of latent ability using a response pattern of graded scores. Psychometrika Monograph Supplement. No. 17 (pp. 1–98). Richamond, VA: Psychometric Society. Retrieved from http://www.psychometrika.org/jounal/online/MN17.pdf.
Thissen, D., Varni, J. W., Stucky, B. D., Liu, Y., Irwin, D. E., & Dewalt, D. A. (2011). Using the PedsQL 3.0 asthma module to obtain scores comparable with those of the PROMIS pediatric asthma impact scale (PAIS). Quality of Life Research, 20(9), 1497–1505. doi:10.1007/s11136-011-9874-y.
Kolen, M. J., & Brennan, R. L. (2004). Test equating, scaling, and linking. Methods and Practice (2nd ed.). New York: Springer.
Acknowledgments
PROMIS® was funded with cooperative agreements from the National Institutes of Health (NIH) Common Fund Initiative Northwestern University, PI: David Cella, PhD, U54AR057951, U01AR052177; Northwestern University, PI: Richard C. Gershon, PhD, U54AR057943; American Institutes for Research, PI: Susan (San) D. Keller, PhD, U54AR057926; State University of New York, Stony Brook, PIs: Joan E. Broderick, PhD and Arthur A. Stone, PhD, U01AR057948, U01AR052170; University of Washington, Seattle, PIs: Heidi M. Crane, MD, MPH, Paul K. Crane, MD, MPH, and Donald L. Patrick, PhD, U01AR057954; University of Washington, Seattle, PI: Dagmar Amtmann, PhD, U01AR052171; University of North Carolina, Chapel Hill, PI: Harry A. Guess, MD, PhD (deceased), Darren A. DeWalt, MD, MPH, Bryce B. Reeve, PhD, U01AR052181; Children’s Hospital of Philadelphia, PI: Christopher B. Forrest, MD, PhD, U01AR057956; Stanford University, PI: James F. Fries, MD, U01AR052158; Boston University, PIs: Alan Jette, PT, PhD, Stephen M. Haley, PhD (deceased), and David Scott Tulsky, PhD (University of Michigan, Ann Arbor), U01AR057929; University of California, Los Angeles, PIs: Dinesh Khanna, MD (University of Michigan, Ann Arbor) and Brennan Spiegel, MD, MSHS, U01AR057936; University of Pittsburgh, PI: Paul A. Pilkonis, PhD, U01AR052155; Georgetown University, PIs: Carol. M. Moinpour, PhD (Fred Hutchinson Cancer Research Center, Seattle) and Arnold L. Potosky, PhD, U01AR057971; Children’s Hospital Medical Center, Cincinnati, PI: Esi M. Morgan DeWitt, MD, MSCE, U01AR057940; University of Maryland, Baltimore, PI: Lisa M. Shulman, MD, U01AR057967; and Duke University, PI: Kevin P. Weinfurt, PhD, U01AR052186). NIH Science Officers on this project have included Deborah Ader, PhD, Vanessa Ameen, MD (deceased), Susan Czajkowski, PhD, Basil Eldadah, MD, PhD, Lawrence Fine, MD, DrPH, Lawrence Fox, MD, PhD, Lynne Haverkos, MD, MPH, Thomas Hilton, PhD, Laura Lee Johnson, PhD, Michael Kozak, PhD, Peter Lyster, PhD, Donald Mattison, MD, Claudia Moy, PhD, Louis Quatrano, PhD, Bryce B. Reeve, PhD, William Riley, PhD, Peter Scheidt, MD, Ashley Wilder Smith, PhD, MPH, Susana Serrate-Sztein, MD, William Phillip Tonkins, DrPH, Ellen Werner, PhD, Tisha Wiley, PhD, and James Witter, MD, PhD. The contents of this article uses data developed under PROMIS. These contents do not necessarily represent an endorsement by the US Federal Government or PROMIS. See www.nihpromis.org for additional information on the PROMIS® initiative.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Drs. DeWalt and Tulsky were unpaid members of the Board of Directors for the PROMIS Health Organization (PHO) during the conduct of this study. Drs. Reeve and Tulsky were unpaid members of the Board of Directors for the PHO during the preparation of this manuscript. The remaining authors have no financial relationships or conflicts of interest relevant to this study to disclose.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Reeve, B.B., Thissen, D., DeWalt, D.A. et al. Linkage between the PROMIS® pediatric and adult emotional distress measures. Qual Life Res 25, 823–833 (2016). https://doi.org/10.1007/s11136-015-1143-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-015-1143-z